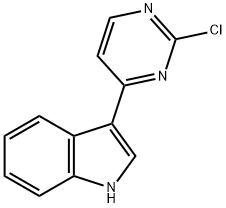
3-(2-chloropyriMidin-4-yl)-1H-indole synthesis
- Product Name:3-(2-chloropyriMidin-4-yl)-1H-indole
- CAS Number:945016-63-7
- Molecular formula:C12H8ClN3
- Molecular Weight:229.66
120-72-9
620 suppliers
$6.00/25g
3934-20-1
709 suppliers
$9.00/1g

945016-63-7
80 suppliers
$44.00/100mg
Yield: 79%
Reaction Conditions:
with toluene-4-sulfonic acid at 110; for 3 h;Inert atmosphere;
Steps:
102.1 Step 1: preparation of 3-(2-chloropyrimidin-4-yl)-1H-indole
3-(2-chloropyrimidin-4-yl)-1H-indole (1 g, 4.37 mmol), 2-(difluoromethoxy)-4-fluoro-5-nitroaniline (810 mg, 4.37mmol) and p-toluenesulfonic acid (750 mg, 4.37 mmol) were dissolved in 2-pentanol (40 mL), and then the reactionsolution was heated at 110 °C for 3 hours. After LCMS showed completion of the reaction, the reaction solution wascooled to room temperature naturally, and a dark solid was precipitated. The solid was filtered, and the filter cake waswashed with methanol and methyl tert-butyl ether to obtain 3-(2-chloropyrimidin-4-yl)-1H-indole (1.3 g, 79%).
References:
Shanghai Hansoh Biomedical Co., Ltd.;Jiangsu Hansoh Pharmaceutical Group Co., Ltd.;WEI, Mingsong;SUN, Guangjun;TAN, Songliang;GAO, Peng;WANG, Shaobao;XIU, Wenhua;ZHANG, Fujun;BAO, Rudi EP3205650, 2017, A1 Location in patent:Paragraph 0106; 0512; 0513
882562-58-5
5 suppliers
inquiry

945016-63-7
80 suppliers
$44.00/100mg
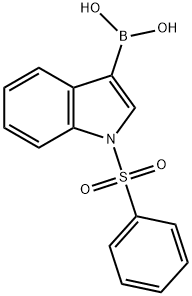
129271-98-3
92 suppliers
$15.00/100mg

945016-63-7
80 suppliers
$44.00/100mg
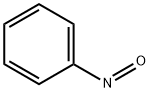
586-96-9
122 suppliers
$20.00/1G

37968-69-7
21 suppliers
$356.00/1g

945016-63-7
80 suppliers
$44.00/100mg