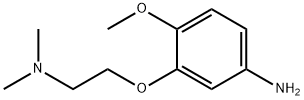
3-[2-(dimethylamino)ethoxy]-4-methoxybenzenamine synthesis
- Product Name:3-[2-(dimethylamino)ethoxy]-4-methoxybenzenamine
- CAS Number:170229-68-2
- Molecular formula:C11H18N2O2
- Molecular Weight:210.27

170229-67-1
0 suppliers
inquiry
![3-[2-(dimethylamino)ethoxy]-4-methoxybenzenamine](/CAS/20180703/GIF/170229-68-2.gif)
170229-68-2
7 suppliers
inquiry
Yield:170229-68-2 98%
Reaction Conditions:
with hydrogen;palladium 10% on activated carbon in ethanol; under 2585.81 Torr;
Steps:
22
To a solution of 2-methoxy-5-nitro-phenol (10 g) , (2-chloro- ethyl) -dimethyl-amine hydrochloride (9.37 g) in dimethylformamide (15OmL) was added NaH (2.98 g) slowly under N2 and at room temperature. The mixture was then stirred at 140 0C heating with an oil bath for 5 hours. The reaction was concentrated to dryness, dissolved in 2 M HCl (200 iuL) and washed with ethyl acetate (2x100 mL) . The aqueous layer was basified with K2CO3, extracted with dichloromethane (3x200 mL) EPO
References:
WO2006/91963,2006,A1 Location in patent:Page/Page column 106-107
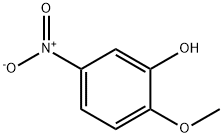
636-93-1
263 suppliers
$6.00/5g
![3-[2-(dimethylamino)ethoxy]-4-methoxybenzenamine](/CAS/20180703/GIF/170229-68-2.gif)
170229-68-2
7 suppliers
inquiry