
3-(2-PHENOXYPHENYL)PROPANOIC ACID synthesis
- Product Name:3-(2-PHENOXYPHENYL)PROPANOIC ACID
- CAS Number:40492-92-0
- Molecular formula:C15H14O3
- Molecular Weight:242.27

915034-53-6
0 suppliers
inquiry

40492-92-0
22 suppliers
$35.00/250mg
Yield:40492-92-0 98%
Reaction Conditions:
Stage #1: C17H18O3with lithium hydroxide in tetrahydrofuran; for 5 h;Heating / reflux;
Stage #2: with hydrogenchloride in tetrahydrofuran;water;ethyl acetate at 20;
Steps:
A solution of 2-phenoxybenzoic acid (660 mg, 3.08 mmoles) and N,O-dimethylhydroxylamine hydrochloride (451 mg, 4.62 mmoles) in DMF (15 ml) was treated at rt for 18 h with triethylamine (1.3 ml, 9.24 mmoles) and HBTU (1.75 g, 4.62 mmoles). The reaction mixture was partitioned between 1N NaOH and ethyl acetate. The organic layer was washed with brine, dried over sodium sulfate and concentrated to afford 1 which was not further purified (827 mg, 100%). A 1M solution of lithium aluminum hydride in THF (3.18 ml, 3.18 mmoles) was slowly added to a cold (-20° C.) solution of 1 (410 mg, 1.59 mmoles) in THF (8 ml). The solution was stirred at -20° C. for 45 min and then quenched slowly with water. The mixture was filtered through a cake of celite and the filtrate was partitioned between ethyl acetate and saturated bicarbonate. The organic layer was washed with brine, dried over sodium sulfate and concentrated to afford aldehyde 2 (278 mg, 88%) which was not further purified. A solution of triethylphosphonoacetate (0.334 ml, 1.68 mmoles) in THF (2 ml) was treated with 60% sodium hydride (132 mg, 3.3 mmoles) at rt for 10 min. The aldehyde 2 (278 mg, 1.40 mmoles) was then added and the solution was stirred at rt for 4 h. The reaction mixture was partitioned between 1 N HCl and ethyl acetate. The organic layer was washed successively with water, saturated sodium carbonate and brine, then dried over sodium sulfate, filtered and concentrated. The crude was adsorbed on silica and purified on a silica gel column with a 5-10% ethyl acetate/hexanes gradient to afford ester 3 (258 mg, 59%). A solution of ester 3 (136 mg, 0.51 mmoles) in EtOH (5 ml) was placed in the Parr apparatus. A catalytic amount of 10% Pd/C (25 mg) was added and the mixture was shaken under an atmosphere of hydrogen (50 psi) for 1.5 h. The heterogeneous mixture was filtered through a cake of celite and concentrated to afford ester 4 (127 mg, 93%). This ester (127 mg, 0.47 mmoles) was dissolved in THF (10 ml)/ water (5 ml) and treated with lithium hydroxide monohydrate (197 mg, 4.7 mmoles) for 5 h at reflux. The mixture was cooled to rt and partitioned between 1N HCl and ethyl acetate. The organic layer was washed with water and brine then dried over sodium sulfate and concentrated to afford the desired acid (112 mg, 98%).
References:
US2006/258740,2006,A1 Location in patent:Page/Page column 11-12
108-86-1
508 suppliers
$10.00/5g
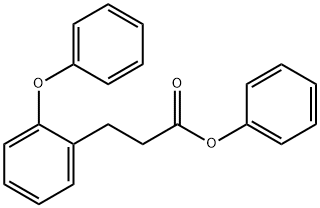
301346-12-3
0 suppliers
inquiry

495-78-3
142 suppliers
$10.00/1g

40492-92-0
22 suppliers
$35.00/250mg