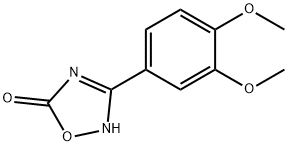
3-(3,4-Dimethoxyphenyl)-1,2,4-oxadiazol-5-ol synthesis
- Product Name:3-(3,4-Dimethoxyphenyl)-1,2,4-oxadiazol-5-ol
- CAS Number:26904-02-9
- Molecular formula:C10H10N2O4
- Molecular Weight:222.2
Yield:26904-02-9 800 mg
Reaction Conditions:
with 1,8-diazabicyclo[5.4.0]undec-7-ene in 1,4-dioxane at 15 - 110; for 12 h;
Steps:
6.3 Step 3: Preparation of 3-(3, 4-dimethoxyphenyl)- 1,2, 4-oxadiazol-5(4H)-one.
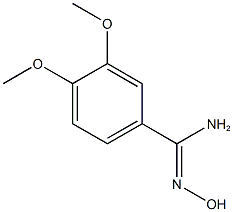
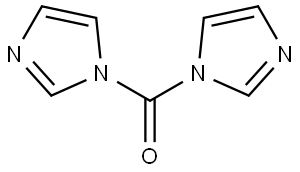
A mixture of N-hydroxy-3,4-dimethoxybenzimidamide (1.0 g, 5.10 mmol), 1,8- diazabicyclo[5.4.0]undec-7-ene (853 mg, 5.61 mmol, 845 iL) and 1,1 ‘-carbonyldiimidazole (1 .24 g, 7.65 mmol) in dioxane (10 mL) was prepared at 15°C. The mixture was warmed to 110°C for 12 h. The mixture was cooled to 1 5°C and then poured into water (5 mL). The aqueous phase was extracted with dichloromethane (1 0 mL x 5), then the combined organic phases were washed with saturated aqueoussodium chloride solution (5 mL), dried over anhydrous sodium sulfate, filtered and concentrated in vacuo to afford 3-(3,4-dimethoxyphenyl)-1 ,2,4-oxadiazol-5(4H)-one (800 mg) as a yellow solid. 1 NMR (400MHz, 0D013) O 7.60-7.52 (m, 2H), 6.88 (d, J 8.8 Hz, 1H), 3.91 (d, J 6.1 Hz, 6H); LCMS (ESI) m/z223.2 [M÷H]÷.
References:
WO2018/81167,2018,A1 Location in patent:Page/Page column 98; 99