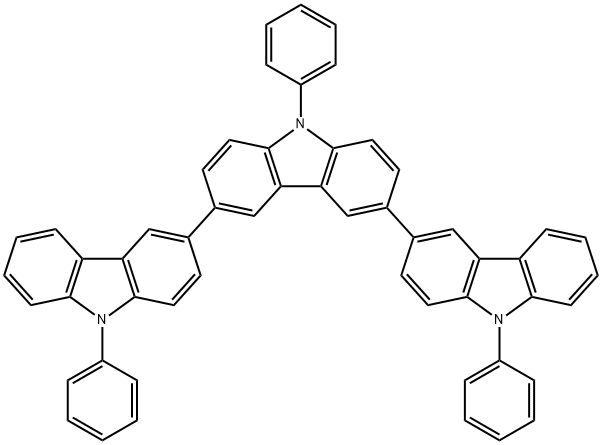
3,3':6',3''-Ter-9H-carbazole, 9,9',9''-triphenyl- synthesis
- Product Name:3,3':6',3''-Ter-9H-carbazole, 9,9',9''-triphenyl-
- CAS Number:1141757-83-6
- Molecular formula:C54H35N3
- Molecular Weight:725.88
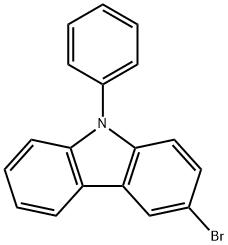
1153-85-1
318 suppliers
$7.00/1g
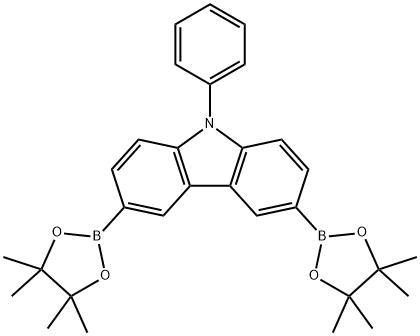
618442-57-2
86 suppliers
$16.00/250mg

1141757-83-6
49 suppliers
inquiry
Yield:1141757-83-6 82%
Reaction Conditions:
Stage #1: 3-bromo-9-phenyl-9H-carbazole;9-phenyl-3,6-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-9H-carbazolewith potassium carbonate in water;toluene at 20; for 0.5 h;
Stage #2: with tetrakis(triphenylphosphine) palladium(0) in water;toluene; for 24 h;Reflux;
Steps:
1.1 Step 1) Preparation of Compound 1
(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl) -9-phenylcarbazole,One equivalent (20 g, 40.49 mmol),3-Bromo-9-phenylcarbazole, 2Equivalent (26 g, 81 mmol), 33.5 g of K2CO3, 800 ml of toluene,And 200 ml of water was added to a 2-L 2-neck round bottom flask. The mixture was stirred at room temperature for 30 minutes,Pd (PPh3) 4 (1.4 g, 1.21 mmol) was added and refluxed for 24 hours.After the reaction was completed, the reaction mixture was extracted with methylene chloride, added with MgSO 4 and filtered.After removing the solvent of the obtained organic layer, it was purified by column chromatography to obtain intermediate compound 1 (24.80 g, yield 82%).
References:
KR101847236,2018,B1 Location in patent:Paragraph 0066-0070

1126522-69-7
129 suppliers
$10.00/1g

57103-20-5
239 suppliers
$5.00/1g

1141757-83-6
49 suppliers
inquiry