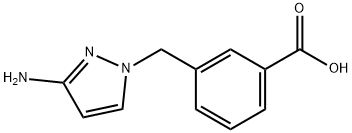
3-[(3-amino-1H-pyrazol-1-yl)methyl]benzoic acid synthesis
- Product Name:3-[(3-amino-1H-pyrazol-1-yl)methyl]benzoic acid
- CAS Number:1003013-01-1
- Molecular formula:C11H11N3O2
- Molecular Weight:217.22
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
![3-[(3-amino-1H-pyrazol-1-yl)methyl]benzoic acid](/CAS/20180601/GIF/1003013-01-1.gif)
1003013-01-1
6 suppliers
inquiry
Yield: 63%
Reaction Conditions:
Stage #1:3-(3-amino-pyrazol-1-ylmethyl)-benzoic acid methyl ester with sodium hydroxide;water in tetrahydrofuran;methanol at 25; for 1.5 h;
Stage #2: with hydrogenchloride in tetrahydrofuran;methanol;water; pH=~ 7.5
Stage #3: with citric acid in tetrahydrofuran;methanol;water; pH=~ 7
Steps:
63
The 3-(3-amino-pyrazol-1-ylmethyl)-benzoic acid methyl ester (prepared in example 55, 900 mg, 3.89 mmol) was dissolved in tetrahydrofuran (6 mL) and methanol (6 mL) was added. A 3.0 N aqueous sodium hydroxide solution (6 mL) was added and the reaction stirred at 25° C. for 1.5 h. 1.0 M aqueous hydrochloric acid solution was added to adjust the pH to 7.5 followed by the addition of 10% aqueous citric acid to adjust the pH to 7. The product was extracted into ethyl acetate and the combined organic layers were dried over magnesium sulfate and concentrated in vacuo to give the desired product, 3-(3-amino-pyrazol-1-ylmethyl)-benzoic acid (536 mg, 63%) as a white solid: H1-NMR (400 MHz, DMSO-d6) δ 5.08 (2H, s), 5.41 (1H, d, J=2.0 Hz), 7.41-7.43 (2H, m), 7.45 (1H, d, J=2.4 Hz), 7.73 (1H, s), 7.79-7.82 (1H, m).
References:
Berthel, Steven Joseph;Kester, Robert Francis;Murphy, Douglas Eric;Prins, Thomas Jay;Ruebsam, Frank;Sarabu, Ramakanth;Tran, Chinh Viet;Vourloumis, Dionisios US2008/21032, 2008, A1 Location in patent:Page/Page column 60
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
![3-[(3-amino-1H-pyrazol-1-yl)methyl]benzoic acid](/CAS/20180601/GIF/1003013-01-1.gif)
1003013-01-1
6 suppliers
inquiry
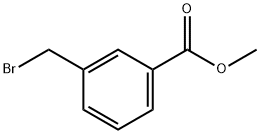
1129-28-8
239 suppliers
$13.43/1gm:
![3-[(3-amino-1H-pyrazol-1-yl)methyl]benzoic acid](/CAS/20180601/GIF/1003013-01-1.gif)
1003013-01-1
6 suppliers
inquiry