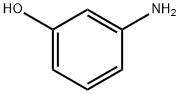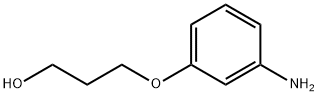
3-(3-AMinophenoxy)-1-propanol synthesis
- Product Name:3-(3-AMinophenoxy)-1-propanol
- CAS Number:121486-70-2
- Molecular formula:C9H13NO2
- Molecular Weight:167.21
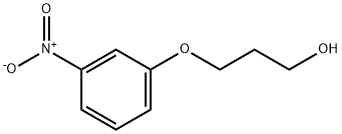
63082-35-9
6 suppliers
inquiry

121486-70-2
4 suppliers
inquiry
Yield:121486-70-2 82%
Reaction Conditions:
with tin(II) chloride dihdyrate in ethanol; for 3 h;Reflux;Inert atmosphere;
Steps:
6 4.2.1.2. General procedure II (nitro reduction)
General procedure: To a solution of nitrobenzene in EtOH (0.1M) was added 4 equiv of SnCl2·2H2O. The reaction mixture was refluxed for 3 h and concentrated in vacuo. The residue was diluted with EtOAc and saturated NaHCO3 solution was added. The mixture was filtered using a Celite pad. The organic phase was washed with water and brine, dried over MgSO4, and concentrated in vacuo. Purification of the residue via flash column chromatography on silica gel afforded the corresponding aniline. 4.2.31. 3-(3-(4,6-Bis(4-chlorophenyl)pyrimidin-2-ylamino)-phenoxy)propan-1-ol (14); Aminopyrimidine 14 (93 mg, 67%) was prepared, via general procedure III, from 3-(3-aminophenoxy)propan-1-ol (50 mg, 0.30 mmol), which was prepared from nitrobenzene 13 (82%) via general procedure II;
References:
Han, Young Taek;Kim, Kyeojin;Son, Dohyun;An, Hongchan;Kim, Hee;Lee, Jeeyeon;Park, Hyun-Ju;Lee, Jeewoo;Suh, Young-Ger [Bioorganic and Medicinal Chemistry,2015,vol. 23,# 3,p. 579 - 587]
![Acetamide, N-[3-(3-hydroxypropoxy)phenyl]-](/CAS/20180529/GIF/666174-09-0.gif)
666174-09-0
0 suppliers
inquiry

121486-70-2
4 suppliers
inquiry