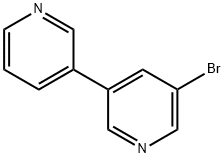
[3,3'-Bipyridin]-5-ylboronic acid synthesis
- Product Name:[3,3'-Bipyridin]-5-ylboronic acid
- CAS Number:1034541-67-7
- Molecular formula:C10H9BN2O2
- Molecular Weight:200
Yield:1034541-67-7 37%
Reaction Conditions:
Stage #1: Triisopropyl borate;5-bromo-3,3'-bipyridinewith n-butyllithium in tetrahydrofuran;hexanes;toluene at -40 - -20; for 0.5 h;
Stage #2: with hydrogenchloride;water in tetrahydrofuran;hexanes;toluene at -20 - 20;
Stage #3: with sodium hydroxide;water in tetrahydrofuran;hexanes;toluene; pH=7.6;
Steps:
2
Compound 20 - A three-necked round-bottomed flask equipped with a thermometer was charged with compound 19 (1.2 g, 5.1 mmol), toluene (8 mL), THF (3 mL), and trisopropylborate (1.4 mL, 6.0 mmol). After cooling to - 40 0C (dry ice/acetone),
References:
WO2008/137604,2008,A1 Location in patent:Page/Page column 4; 31-32; 40
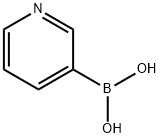
1692-25-7
481 suppliers
$5.00/1g
![[3,3'-Bipyridin]-5-ylboronic acid](/CAS/20180703/GIF/1034541-67-7.gif)
1034541-67-7
22 suppliers
inquiry
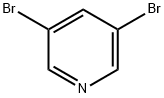
625-92-3
480 suppliers
$6.00/10g
![[3,3'-Bipyridin]-5-ylboronic acid](/CAS/20180703/GIF/1034541-67-7.gif)
1034541-67-7
22 suppliers
inquiry