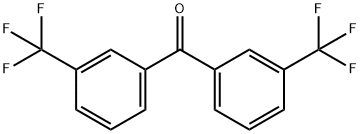
3,3'-BIS(TRIFLUOROMETHYL)BENZOPHENONE synthesis
- Product Name:3,3'-BIS(TRIFLUOROMETHYL)BENZOPHENONE
- CAS Number:1868-00-4
- Molecular formula:C15H8F6O
- Molecular Weight:318.21

401-78-5
393 suppliers
$5.00/5g
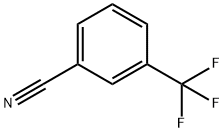
368-77-4
271 suppliers
$10.00/10 g

1868-00-4
99 suppliers
$11.00/250mg
Yield:-
Reaction Conditions:
with hydrogenchloride;n-butyllithium;sodium hydrogencarbonate in hexane;water
Steps:
82 3,3'-bis(Trifluoromethyl)benzophenone
EXAMPLE 82 3,3'-bis(Trifluoromethyl)benzophenone To a stirred solution of n-butyl lithium in hexane (1.6M; 100 mL) at -78° C. under argon, was added dropwise a solution of 3-bromobenzotrifluoride (36 g) in dry ether (75 mL) over 15 minutes. After the addition was complete, the solution was stirred at -78° C. for 45 minutes, then a solution of alpha,alpha,alpha-trifluoro-m-toluonitrile (25.7 g) in dry ether was added over 20 minutes. The deep red mixture was allowed to react at -78° C. for 30 minutes, then immediately after the cooling bath was removed 6N hydrochloric acid (100 mL) was added in a rapid stream. The reaction mixture decolorized and a dense precipitate formed as the reaction temperature rose rapidly to 0°-5° C. Water (200 mL) and ether (200 mL) were added, and the reaction was warmed to 35° C. and was stirred at that temperature until the precipitate dissolved (2 hours). After the reaction was cooled, the layers were separated and the aqueous phase was washed with ether (200 mL). The organic extracts were washed in turn with saturated sodium bicarbonate, then were combined, dried (MgSO4) and evaporated. The solid residue was dissolved in hot hexane (200 mL) and the mixture was left at 0°-5° C. overnight. The resulting colorless crystalline material was filtered, washed with hexane and dried to give 43.9 g of 3,3'-bis(trifluoromethyl)benzophenone mp 98.5°-99.5° C.
References:
Hoffmann-La Roche Inc. US4788206, 1988, A
1598-89-6
31 suppliers
$54.00/1g

1868-00-4
99 suppliers
$11.00/250mg

79-37-8
463 suppliers
$17.67/10gm:
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

1868-00-4
99 suppliers
$11.00/250mg

79-37-8
463 suppliers
$17.67/10gm:
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

1868-00-4
99 suppliers
$11.00/250mg

401-78-5
393 suppliers
$5.00/5g

1868-00-4
99 suppliers
$11.00/250mg