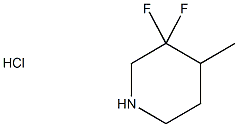
3,3-Difluoro-4-Methylpiperidine Hydrochloride synthesis
- Product Name:3,3-Difluoro-4-Methylpiperidine Hydrochloride
- CAS Number:374794-78-2
- Molecular formula:C6H12ClF2N
- Molecular Weight:171.62
374794-77-1
54 suppliers
$151.00/250mg

374794-78-2
33 suppliers
$45.00/2.5mg
Yield:374794-78-2 78%
Reaction Conditions:
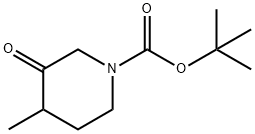
Stage #1: tert-butyl 4-methyl-3-oxopiperidine-1-carboxylatewith diethylamino-sulfur trifluoride in dichloromethane at 0 - 20; for 16 h;
Stage #2: with water in dichloromethane at 20; for 0.333333 h;
Stage #3: with hydrogenchloride in methanol;diethyl ether at 20; for 0.5 h;
Steps:
39 DESCRIPTION 39; (RS)-3,3-Difluoro-4-methylpiperidine Hydrochloride
Diethylaminosulphur trifluoride (1.18 mL, 8.97 mmol) was added to a stirred, cooled (020 C.) solution of (RS)-1,1-dimethylethyl 4-methyl-3-oxopiperidinecarboxylate (Description 38, 500 mg, 2.24 mmol) in dichloromethane and the mixture was stirred at room temperature for 16 hours. Ice (5 g) and water (5 mL) were added and the mixture was stirred at room temperature for 20 minutes. The layers were separated and the aqueous layer was extracted with dichloromethane (2×50 mL). The combined organic fractions were washed with brine (50 mL), dried (MgSO4) and the solvent was evaporated under reduced pressure. The residue was suspended in diethyl ether (50 mL) and treated with methanolic hydrogen chloride (1M, 3 mL). The mixture was stirred at room temperature for 30 minutes, then the solvent was evaporated under reduced pressure to give the title compound as a solid (303 mg, 78%). m/z (ES+) 136 (M+1).
References:
US2003/225059,2003,A1 Location in patent:Page 26-27
7647-01-0
11 suppliers
$10.00/10g

374794-77-1
54 suppliers
$151.00/250mg

374794-78-2
33 suppliers
$45.00/2.5mg