
3,3-Dimethyl-1,5-pentanediol synthesis
- Product Name:3,3-Dimethyl-1,5-pentanediol
- CAS Number:53120-74-4
- Molecular formula:C7H16O2
- Molecular Weight:132.2

4839-46-7
221 suppliers
$11.00/5g

53120-74-4
22 suppliers
$65.00/10mg
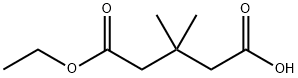
71885-49-9
5 suppliers
inquiry
Yield:53120-74-4 96.2%
Reaction Conditions:
with sulfuric acid;sodium hydrogencarbonate in methanol;water;1,2-dichloro-ethane;
Steps:
40.i i)
i) Synthesis of 3,3-dimethyl-1,5-pentanediol To a solution of 13.38 g (83.5 mmol) of 3,3-dimethylglutaric acid and 90 ml (60 mmol) of methanol in 200 ml of 1,2-dichloroethane was added 4.18 ml of conc. sulfuric acid at room temperature. The mixture was heated for 16 hours under reflux. The reaction mixture was cooled, to which was added water. The organic layer was separated, washed with an aqueous solution of sodium hydrogencarbonate and dried. The solvent was distilled off, and the residue was purified by column chromatography (eluent: n-hexane/ethyl acetate=2:1) to give ethyl 3,3-dimethyl glutarate. This product was added, at room temperature, to a suspension of 3.80 g (100 mmol) of lithium aluminum hydride in 250 ml of tetrahydrofuran. The mixture was stirred for 16 hours. Water was added to this mixture until excess amount of lithium aluminum hydride was decomposed. The organic layer was dried, and the resulting precipitate was filtered off. The solvent was then distilled off to give 10.62 g of the desired compound (96.2%, white crystals). NMR(200 MHz,CDCl3) δ: 0.95(6H,s), 1.58(4H,t, J=7.0 Hz), 3.74(4H,t,J=7.0 Hz).
References:
US5958942,1999,A

4839-46-7
221 suppliers
$11.00/5g

53120-74-4
22 suppliers
$65.00/10mg
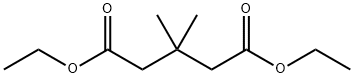
17804-59-0
7 suppliers
inquiry

53120-74-4
22 suppliers
$65.00/10mg
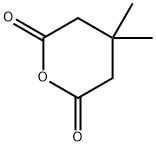
4160-82-1
120 suppliers
$38.20/5g

53120-74-4
22 suppliers
$65.00/10mg

4839-46-7
221 suppliers
$11.00/5g

53120-74-4
22 suppliers
$65.00/10mg

17804-59-0
7 suppliers
inquiry