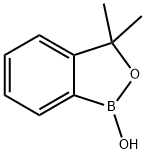
3,3-diMethylbenzo[c][1,2]oxaborol-1(3H)-ol synthesis
- Product Name:3,3-diMethylbenzo[c][1,2]oxaborol-1(3H)-ol
- CAS Number:221352-10-9
- Molecular formula:C9H11BO2
- Molecular Weight:161.99
![3,3-diMethylbenzo[c][1,2]oxaborol-1(3H)-ol 3,3-diMethylbenzo[c][1,2]oxaborol-1(3H)-ol](/NewsImg/2023-10-17/6383313715250298005052562.jpg)

7073-69-0
87 suppliers
$22.00/250mg
![3,3-diMethylbenzo[c][1,2]oxaborol-1(3H)-ol](/CAS/GIF/221352-10-9.gif)
221352-10-9
76 suppliers
$40.00/100mg
Yield:221352-10-9 82.4%
Reaction Conditions:
Stage #1:2-(2-bromophenyl)propanol with methylmagnesium bromide in tetrahydrofuran at 0;Cooling with ice;
Stage #2: with n-butyllithium in tetrahydrofuran;hexane at -40;Cooling with acetone-dry ice;
Stage #3: with Triisopropyl borate in tetrahydrofuran;hexane at 20;Product distribution / selectivity;
Steps:
82
82c[0384] A 50 mL round-bottomed-flask equipped with a magnetic stir bar and ice- H2O bath was charged with 82b (860 mg, 4.0 mmol) and anhydrous THF (20 mL). MeMgBr (1.3 mL, 2.0 M in THF) was slowly added via a syringe. The mixture was stirred at 0 0C for 10 minute and the ice bath was replaced with a dry ice-acetone bath at -40 0C. BuLi (1.9 mL, 2.5 M in hexanes) was added dropwise via a syringe. The resulting mixture was stirred at -40 0C for another 2h before B(O-ipr)3 (1.4 mL, 4.8 mmol) was added dropwise. The mixture was allowed to warm up to rt gradually and stirred overnight. After carefully quenched the reaction with H2O (1 mL), HCl (3M, 10 mL) was added and the mixture was stirred at rt for Ih. The mixture was extracted with EtOAc (20 mL) 3 times. Combined extracts was washed with H2O (20 mL), brine (20 mL), dried over MgSO4, filtered and concentrated under reduced pressure to give a clear oil. The oil solidified overnight to give 82c as a pale yellow waxy solid (544mg, 82.4%).
References:
ANACOR PHARMACEUTICALS, INC.;CHEN, Daitao;ORR, Matthew;SLIGAR, Jessica;JACOBS, Robert;PLATTNER, Jacob, J. WO2011/19618, 2011, A1 Location in patent:Page/Page column 137-138

5419-55-6
372 suppliers
$12.00/5g

7073-69-0
87 suppliers
$22.00/250mg
![3,3-diMethylbenzo[c][1,2]oxaborol-1(3H)-ol](/CAS/GIF/221352-10-9.gif)
221352-10-9
76 suppliers
$40.00/100mg
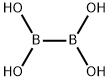
13675-18-8
157 suppliers
$6.00/1g

7073-69-0
87 suppliers
$22.00/250mg
![3,3-diMethylbenzo[c][1,2]oxaborol-1(3H)-ol](/CAS/GIF/221352-10-9.gif)
221352-10-9
76 suppliers
$40.00/100mg
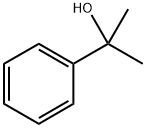
617-94-7
184 suppliers
$6.00/1g
![3,3-diMethylbenzo[c][1,2]oxaborol-1(3H)-ol](/CAS/GIF/221352-10-9.gif)
221352-10-9
76 suppliers
$40.00/100mg
1008106-85-1
9 suppliers
inquiry

67-64-1
6 suppliers
$17.30/10ml
![3,3-diMethylbenzo[c][1,2]oxaborol-1(3H)-ol](/CAS/GIF/221352-10-9.gif)
221352-10-9
76 suppliers
$40.00/100mg