
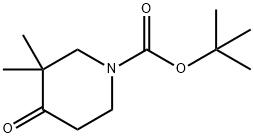
3,3-DIMETHYLPIPERIDIN-4-OL synthesis
- Product Name:3,3-DIMETHYLPIPERIDIN-4-OL
- CAS Number:373603-88-4
- Molecular formula:C7H15NO
- Molecular Weight:129.2
324769-06-4
147 suppliers
$9.00/250mg

373603-88-4
48 suppliers
$37.00/100mg
Yield:373603-88-4 38 mg
Reaction Conditions:
Stage #1: tert-butyl 3,3-dimethyl-4-oxopiperidine-1-carboxylatewith methanol;sodium tetrahydroborate at 25; for 12 h;
Stage #2: with trifluoroacetic acid in dichloromethane at 20; for 12 h;
Steps:
Intermediate C2c: 3,3-dimethylpiperidin-4-ol
To a solution of tert- butyl 3,3-dimethyl-4-oxo-piperidine-1-carboxylate (160 mg, 0.70 mmol) in methanol (8 ml_) was added portionwise sodium borohydride (80 mg, 2.11 mmol). The solution was stirred at 25 °C for 12h. Water (1 ml_) was added to quench the excess sodium borohydride. After all volatiles were removed, the crude was dried under high vacuum for 3h. DCM (10 ml_) was then added to the crude, followed by trifluoroacetic acid (0.7 ml_). The resulting solution was stirred at rt for 12h. Crude product was purified using an SCX-2 (2G) column to give the title compound (38 mg) as a colourless oil, which was used without further purification. 1 H NMR (500 MHz, methanol-d4) d 3.34 (dd, J = 10.2, 4.4 Hz, 1 H), 3.33 - 3.31 (m, 1 H), 3.01 (dtd, J = 13.1 , 4.2, 1.6 Hz, 1 H), 2.62 (dd, J = 13.1 , 1.6 Hz, 1 H), 2.60 - 2.52 (m, 1 H), 1.67 (dq, J = 13.1 , 4.2 Hz, 1 H), 1.55 (dddd, J = 13.1 , 11.0, 10.1 , 4.4 Hz, 1 H), 0.94 (s, 3H), 0.93 (s, 3H).
References:
WO2020/104820,2020,A1 Location in patent:Paragraph 00279