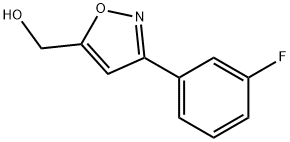
[3-(3-FLUORO-PHENYL)-ISOXAZOL-5-YL]-METHANOL synthesis
- Product Name:[3-(3-FLUORO-PHENYL)-ISOXAZOL-5-YL]-METHANOL
- CAS Number:954240-02-9
- Molecular formula:C10H8FNO2
- Molecular Weight:193.17

107-19-7
5 suppliers
$12.00/25g
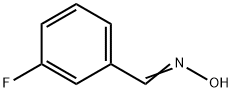
458-02-6
24 suppliers
$75.00/500mg
![[3-(3-FLUORO-PHENYL)-ISOXAZOL-5-YL]-METHANOL](/CAS/GIF/954240-02-9.gif)
954240-02-9
24 suppliers
$240.00/100mg
Yield:-
Reaction Conditions:
Stage #1: 3-fluorobenzaldehyde oximewith pyridine;N-chloro-succinimide in tetrahydrofuran at 60; for 0.666667 h;Inert atmosphere;
Stage #2: propargyl alcoholwith triethylamine in tetrahydrofuran at 50; for 2 h;Inert atmosphere;
Steps:
Gerneral Procedure for Synthesis of Building Block 3 (when R2= 2,3-Dihydrobenzo[b][1,4]dioxin-6-yl).
General procedure: To a solution of 2,3-dihydrobenzo[b][1,4]dioxine-6-carbaldehyde oximes 10 (2.69 g, 15.0 mmol) in THF (70 mL) at room temperature under nitrogen atmosphere, N-chlorosuccinimide (2.40 g, 18.0 mmol) and pyridine (120 μL, 1.50 mmol) were added. After being stirred for 40 min at 60 °C, the mixture was cooled to rt and solutions of propargyl alcohol (700 μL, 12.0 mmol) in THF (2 mL) and triethylamine (2.50 mL, 18.0 mmol) in THF (4 mL) were added dropwise successively. After the mixture being stirred at 50 °C for 2 h. Saturated NaHCO3 solution was added and the mixture was extracted with ethyl acetate (50 mL × 3). The organic layers were dried over anhydrous MgSO4 and concentrated. The residue was purified by flash column chromatography (hexane:EtOAc =2:1) to give the isoxazole alcohol 11 (when R2= 2,3-dihydrobenzo[b][1,4]dioxin-6-yl, 1.87 g, 67%). To a solution of thus prepared alcohol 11 (1.16 g, 5.00mmol) in dichloromethane (15 mL) at room temperature, pyridinium chlorochromate (2.16 g, 10.0 mmol) and 4 ? molecular sieve (4.00 g) were added. After being stirred for 4 h, the mixture was concentrated and the residue was purified by flash column chromatography (hexane:EtOAc =3:1) to give the building block 3 (when R2= 2,3-dihydrobenzo[b][1,4]dioxin-6-yl, 740 mg, 65%). 1H NMR (500 MHz, CDCl3) δ 9.95 (s, 1H), 7.31 (s, 1H), 7.27 (d, J = 8.0 Hz, 1H), 7.13 (s, 1H), 6.90 (d, J = 8.0 Hz,1H), 4.22-4.27 (m, 4H). Other isoxazole aldehyde derivatives were synthesizedanalogously and identified with the 1H NMR spectroscopy.
References:
Oh, Yoo Na;Kwak, Jumyung;Koh, Hun Yeong;Jung, Sun Ho [Bulletin of the Korean Chemical Society,2012,vol. 33,# 12,p. 4227 - 4230]

6175-54-8
0 suppliers
inquiry

458-02-6
24 suppliers
$75.00/500mg
![[3-(3-FLUORO-PHENYL)-ISOXAZOL-5-YL]-METHANOL](/CAS/GIF/954240-02-9.gif)
954240-02-9
24 suppliers
$240.00/100mg
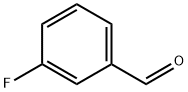
456-48-4
391 suppliers
$9.00/25g
![[3-(3-FLUORO-PHENYL)-ISOXAZOL-5-YL]-METHANOL](/CAS/GIF/954240-02-9.gif)
954240-02-9
24 suppliers
$240.00/100mg