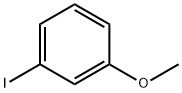
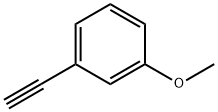
3-(3-METHOXYPHENYL)PROP-2-YNOIC ACID synthesis
- Product Name:3-(3-METHOXYPHENYL)PROP-2-YNOIC ACID
- CAS Number:7621-89-8
- Molecular formula:C10H8O3
- Molecular Weight:176.17

124-38-9
121 suppliers
$214.00/14L
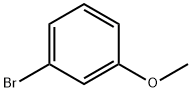
768-70-7
142 suppliers
$12.00/250mg

7621-89-8
16 suppliers
inquiry
Yield:7621-89-8 90%
Reaction Conditions:
Stage #1: carbon dioxide;1-ethynyl-3-methoxybenzenewith potassium carbonate;copper(l) chloride in N,N-dimethyl-formamide at 20; under 760.051 Torr; for 18 h;
Stage #2: with hydrogenchloride in dichloromethane;water; pH=1;Product distribution / selectivity;
Steps:
Table 5. Copper catalyzed carboxylation of terminal alkynes with CCv Reaction conditions: for LI, CuCl (2.0 mol %), TMEDA, 1.5 mol %; for L12, P(NHC)(NHC-Cu), 5 mol%; alkynes (2.0 mmol), base (2.4 mmol), C02 (1 atm), DMF (4 mL), r.t.. M monocarboxylic acid was obtained. General Procedure for Carboxylation of the Terminal Alkynes (lb as Example); CuCl (4.0 mg, 0.04 mmol, 2.0 mol %), TMEDA (3.5 mg, 0.03 mmol, 1.5 mol %), and K2C03 or Cs2C03 (2.4 mmol) were added to the DMF (4 mL) in the reaction tube (10 mL). C02 and 2 mmol of terminal alkynes (la, 204 mg) were introduced into the reaction mixture under stirring. The reaction mixture was stirred at room temperature (about 24 °C) for 16 hours. After completion of the reaction, the reaction mixture was transferred to the potassium carbonate solution (2 N, 5 mL) and the mixture was stirred for 30 mins. The mixture was extracted with dichloromethane (3 >< 5 mL) and the aqueous layer was acidified with concentrated HC1 to PH = 1 , then extracted with diethyl ether (3 χ 5 mL) again. The combined organic layers were dried with anhydrous Na2S04, filtered and the solution was concentrated in vacuum affording pure product. Element analysis calcd (%) for lb [C9H602 (146.0)]: C 73.97, H 4.14; found: C 73.82, H 4.07. Data for 1H and 13C NMR of acids were all conducted in d - DMSO or CDC13 and consistent with the data in reported literatures.
References:
WO2011/75087,2011,A1 Location in patent:Page/Page column 17-18; 26
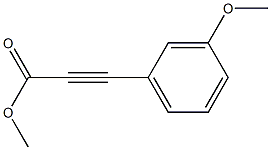
7515-24-4
2 suppliers
inquiry

7621-89-8
16 suppliers
inquiry
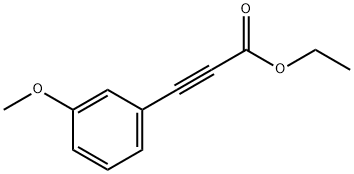
58686-72-9
5 suppliers
inquiry

7621-89-8
16 suppliers
inquiry