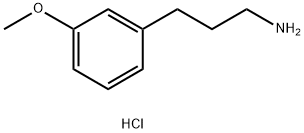
3-(3-Methoxyphenyl)propan-1-amine hydrochloride synthesis
- Product Name:3-(3-Methoxyphenyl)propan-1-amine hydrochloride
- CAS Number:1049696-27-6
- Molecular formula:C10H16ClNO
- Molecular Weight:201.69
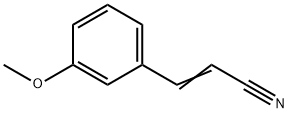
28446-69-7
6 suppliers
inquiry

1049696-27-6
6 suppliers
inquiry
Yield:1049696-27-6 93%
Reaction Conditions:
with hydrogenchloride;palladium 10% on activated carbon;hydrogen in ethanol; for 67 h;
Steps:
4.2.21 4H-Cyclopenta[2,3]pyrrolo[2,1-a][2]benzazepine-2(1H)-one,5,6,11,12,13,13a-hexahydro-8-methoxy-12-methyl-13-(nitromethyl)-(10bR,12S,13R,13aS)-8a
3-Methoxybenzaldehyde (20.8 g, 0.153 mol) was treated with toluene (dry, 140 mL), pyridine (dry, 60 mL), ammonium acetate (0.59 g), and cyanoacetic acid (11.9 g, 0.140 mol) and then heated under reflux with a Dean-Stark trap for 71 h according to the procedure of Montgomery et.al.24 The reaction mixture was concentrated under reduced pressure. The residue was dissolved in CH2Cl2 and washed with 10% aq HCl solution, dried over anhydrous sodium sulfate, and concentrated under reduced pressure. The crude product was purified by flash chromatography on silica gel, eluting with 2-5% EtOAc in hexanes to give 3-methoxycinnamonitrile, 14.65 g (60%). This material was dissolved in EtOH (75 mL) and concd HCl (15 mL) and hydrogenated with 10% Pd/C (2.00 g) at 70 psi on a Parr shaker for 67 h. The reaction mixture was filtered over Celite. The filtrate was extracted with EtOAc, and the EtOAc layer was concentrated under vacuum to give the hydrochloride salt of 3-(3-methoxyphenyl)propylamine as a waxy white solid, 17.3 g (93%).
References:
Goff, Dane A. [Tetrahedron,2013,vol. 69,# 1,p. 242 - 256]
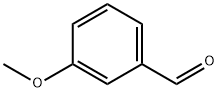
591-31-1
413 suppliers
$10.00/5g

1049696-27-6
6 suppliers
inquiry