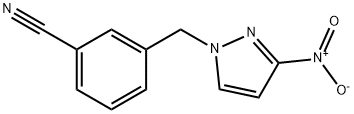
3-[(3-nitro-1H-pyrazol-1-yl)methyl]benzonitrile synthesis
- Product Name:3-[(3-nitro-1H-pyrazol-1-yl)methyl]benzonitrile
- CAS Number:1006569-15-8
- Molecular formula:C11H8N4O2
- Molecular Weight:228.21
26621-44-3
213 suppliers
$6.00/5g

28188-41-2
247 suppliers
$5.00/1g
![3-[(3-nitro-1H-pyrazol-1-yl)methyl]benzonitrile](/CAS/20180601/GIF/1006569-15-8.gif)
1006569-15-8
5 suppliers
inquiry
Yield:1006569-15-8 59%
Reaction Conditions:
Stage #1: 3-nitro-1H-pyrazolewith sodium hydride in N,N-dimethyl-formamide;mineral oil; for 0.5 h;
Stage #2: m-(bromomethyl)benzonitrile in N,N-dimethyl-formamide;mineral oil at 20; for 24 h;
Steps:
12 (S)-N-[1-(3-Cyano-benzyl)-1H-pyrazol-3-yl]-3-cyclopentyl-2-(4-methoxy-2-oxo-2,5-dihydro-pyrrol-1-yl)-propionamide
Example 12 (S)-N-[1-(3-Cyano-benzyl)-1H-pyrazol-3-yl]-3-cyclopentyl-2-(4-methoxy-2-oxo-2,5-dihydro-pyrrol-1-yl)-propionamide A solution of 3-nitropyrazole (prepared as in Example 5, 938 mg, 8.3 mmol) in N,N-dimethylformamide (15 mL) was treated with sodium hydride (519 mg, 60% suspension, 12.97 mmol). Effervescence was observed. The mixture was stirred for 30 min. Then, a solution of 1-bromo-3-cyano-toluene (2.14 g, 10.37 mmol) in N,N-dimethylformamide (5 mL) was added. The mixture was stirred for 24 h at room temperature. The reaction mixture was diluted with water, and extracted with dichloromethane. The organic layer was washed with saturated sodium chloride solution, and dried over magnesium sulfate. The crude product was purified by ISCO flash chromatography (Teledyne Isco RediSep Flash Column 40 g; 0% to 90% ethyl acetate/hexanes) to afford, 3-(3-nitro-pyrazol-1-ylmethyl)-benzonitrile (1. 17 g, 59%), as a white solid.
References:
US2009/264445,2009,A1 Location in patent:Page/Page column 29