
3,4,5,6-tetrahydro-2H-[1,2']bipyridinyl-3'-ylamine synthesis
- Product Name:3,4,5,6-tetrahydro-2H-[1,2']bipyridinyl-3'-ylamine
- CAS Number:5028-14-8
- Molecular formula:C10H15N3
- Molecular Weight:177.25
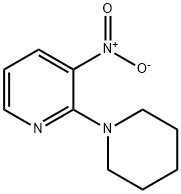
26930-71-2
18 suppliers
inquiry
![3,4,5,6-tetrahydro-2H-[1,2']bipyridinyl-3'-ylamine](/CAS/GIF/5028-14-8.gif)
5028-14-8
21 suppliers
$363.00/1g
Yield:5028-14-8 90.8%
Reaction Conditions:
with palladium 10% on activated carbon;hydrogen in ethanol at 20; for 12 h;
Steps:
2 Step 2: Synthesis of 2-(piperidin-1-yl)pyridin-3-amine (14-4) :
To the ethanolic solution (200 ml) of 3-nitro-2-(piperidin-1-yl)pyridine (1.28g, 6.176mmol) was added dry Pd/C (300 mg). The resulting reaction mixture was kept stirring at room temperature under hydrogen atmosphere for 12 hours. TLC and LCMS of the reaction indicated complete consumption of starting material. The reaction mixture was diluted with ethanol and filtered through a bed of Celite. The filtered reaction mixture was evaporated to get crude material, which was purified by using isolera columns chromatography to afford 2- (piperidin-1-yl)pyridin-3-amine (0.990g, 2.09 mmol, 90.8% yield).
References:
WO2021/3417,2021,A1 Location in patent:Paragraph 00365

220499-46-7
1 suppliers
inquiry
![3,4,5,6-tetrahydro-2H-[1,2']bipyridinyl-3'-ylamine](/CAS/GIF/5028-14-8.gif)
5028-14-8
21 suppliers
$363.00/1g
39856-57-0
294 suppliers
$10.00/1g
![3,4,5,6-tetrahydro-2H-[1,2']bipyridinyl-3'-ylamine](/CAS/GIF/5028-14-8.gif)
5028-14-8
21 suppliers
$363.00/1g

110-89-4
3 suppliers
$15.96/100ML
![3,4,5,6-tetrahydro-2H-[1,2']bipyridinyl-3'-ylamine](/CAS/GIF/5028-14-8.gif)
5028-14-8
21 suppliers
$363.00/1g
16013-85-7
480 suppliers
$9.00/5g
![3,4,5,6-tetrahydro-2H-[1,2']bipyridinyl-3'-ylamine](/CAS/GIF/5028-14-8.gif)
5028-14-8
21 suppliers
$363.00/1g