
3,4,5-TRIMETHYL-4-HEPTANOL synthesis
- Product Name:3,4,5-TRIMETHYL-4-HEPTANOL
- CAS Number:60836-08-0
- Molecular formula:C10H22O
- Molecular Weight:158.28
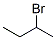
5787-31-5
0 suppliers
inquiry
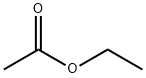
141-78-6
611 suppliers
$22.37/250ml

60836-08-0
4 suppliers
$106.00/250mg
Yield:60836-08-0 51%
Reaction Conditions:
with magnesium;copper(II) oxide in tetrahydrofuran at 65; for 4 h;chemoselective reaction;Barbier Coupling Reaction;
Steps:
Synthesis of tertiary alcohol compounds
General procedure: To a three-necked, 50 mL flaskequipped with a magnetic stirring bar, 15 mL of THF, 2.5 mmol of ester (1 equiv), 0.375 mmol of CuO (15% equiv), 7.5 mmol of aryl halides and magnesium chips (3 equiv) or 15 mmol of alkyl bromides and magnesium chips (6 equiv) were added. The reaction mixture was heated at 65 °C for 4 h. The progress of the reaction was monitored by TLC. Then, the reaction was quenched by saturated aqueous NH4Cl (5 mL), extracted with AcOEt (10 mL × 3), dried over sodium sulfate, filtered, and evaporated to give the raw product. Pure product could be obtained by TLC with petroleum /AcOEt = 5/1 as eluent.
References:
Gao, Fei;Deng, Xiang-Jun;Tang, Yu;Tang, Jin-Peng;Yang, Jun;Zhang, Yuan-Ming [Tetrahedron Letters,2014,vol. 55,# 4,p. 880 - 883] Location in patent:supporting information