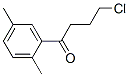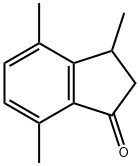
3,4,7-Trimethyl-2,3-dihydro-1H-indene-1-one synthesis
- Product Name:3,4,7-Trimethyl-2,3-dihydro-1H-indene-1-one
- CAS Number:35322-84-0
- Molecular formula:C12H14O
- Molecular Weight:174.24
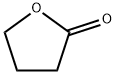
96-48-0
0 suppliers
$24.60/25g

106-42-3
436 suppliers
$19.00/25ML

35322-84-0
2 suppliers
inquiry
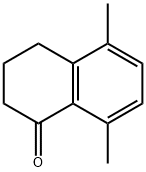
5037-63-8
26 suppliers
$45.00/10mg
Yield:5037-63-8 39.9% ,35322-84-0 2.6%
Reaction Conditions:
in water at 200; for 5 h;Nitrogen atmosphere;
Steps:
1 Example 1:; Silica-carried 10% tungstosilicic acid catalyst, [P-XYLENE]
As a heteropolyacid, tungstosilicic acid (special grade chemical, pure chemistry: [SIO-12WO3-26H2O)] was used as such without being purified. Silica (Fuji Silicia Q- 10, surface area: 270 m2/g, pore diameter: about 10 nm, pore volume: 0.82 cc/g, impurities: Na = 240 ppm, Al = 65 ppm, Ca = 130 ppm, Ti = 100 ppm, total content of impurities = 594 ppm, and therefore silica purity is 99. 9% or higher) was used after firing in a muffle furnace at [500°C] for 5 hours. The process for producing a catalyst will be described.. 6.67 g of tungstosilicic acid was dissolved in 78 ml of pure water and 60 g of fired silica was impregnated with the resulting solution. After air drying, the impregnated silica was dried in a hot-air drying apparatus at [150°C] for 10 hours. The resulting catalyst is referred to as 10 wt% [HSIW/SIO2] catalyst. In a 300 ml stainless steel autoclave equipped with a stirrer, 100 ml of p-xylene (799 mmol) and 1 ml of y- butyrolactone (14.8 mmol) were charged and 3.20 g of the 10 [WT%] [HSIW/SIO2] catalyst (0.097 mmol-HSiW) was charged, and then the autoclave was capped. The autoclave was purged with an accumulated high-purity nitrogen gas and this operation was repeated 10 times. After confirming the absence of pressure loss, the gas was purged. To accelerate temperature raise, the autoclave was dipped in a previously heated oil bath and the reaction was initiated. The reaction was conducted at [200°C] for 5 hours. After the completion of the reaction, the supernatant was removed and then analyzed by an internal standard process using GC (FID, He carrier gas, [30MDB-L] column). After the reaction was conducted for 5 hours, the conversion ratio of [Y-BUTYROLACTONE] was 64.3%, the yield of 5,8-dimethyltetralone based on [Y-BUTYROLACTONE] was 39.9% (TON = 15), and the yield of trimethylindanone was 2.6%
References:
WO2003/101925,2003,A1 Location in patent:Page 17-18
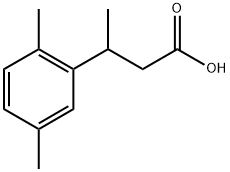
53086-51-4
5 suppliers
inquiry

35322-84-0
2 suppliers
inquiry
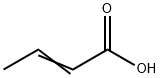
3724-65-0
190 suppliers
$1146.05/25G

106-42-3
436 suppliers
$19.00/25ML

35322-84-0
2 suppliers
inquiry

107-93-7
332 suppliers
$11.19/100G

35322-84-0
2 suppliers
inquiry