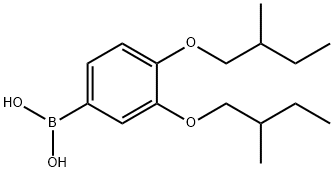
3,4-Bis(2-methylbutyloxy)benzeneboronic acid synthesis
- Product Name:3,4-Bis(2-methylbutyloxy)benzeneboronic acid
- CAS Number:340148-67-6
- Molecular formula:C16H27BO4
- Molecular Weight:294.19
Yield:501434-69-1 98%
Reaction Conditions:
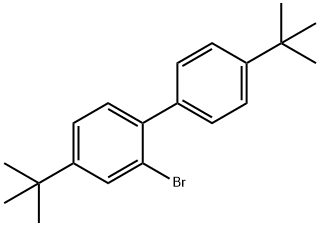
Stage #1: 3,4-bis(2-methylbutyloxy)benzeneboronic acid;2-bromo-4,4'-di-tert-butylbiphenylwith potassium carbonate in water;toluene; for 1 h;
Stage #2: tetrakis(triphenylphosphine) palladium(0) in water;toluene; for 8 h;Heating / reflux;Suzuki Coupling;
Stage #3: with sodium cyanide in water;toluene; for 2 h;
Steps:
A1.2
Preparation of 5'-t-butyl-2'-(4"-t-butylphenyl)-2,3-bis(2-methylbutyloxy)biphenyl 205.5 g (0.595 mol) of 2-bromo-4,4'-di-t-butylbiphenyl, 188.7 g (0.641 mol) of 3,4-bis(2-methylbutyloxy)benzeneboronic acid and 177.2 g (1.282 mol) of K2CO3 were suspended in 840 ml of toluene and 840 ml of H2O and the mixture was saturated with N2 for 1 hour. 1.48 g (1.28 mmol) of Pd(PPh3)4 were subsequently added under protective gas and the mixture was stirred vigorously under reflux for about 8 hours under a blanket of protective gas. 630 ml of 1% strength NaCN solution were added and the mixture was stirred for 2 hours. The organic phase washed three times with water, dried over Na2SO4, filtered and subsequently evaporated completely on a rotary evaporator. This gave 300.2 g (98%) of a light-brown oil which, according to 1H NMR, had a purity of 97% and was used directly in the subsequent reaction. 1H NMR (CDCl3, 500 MHz): [ppm]=7.5-7.3 (m, 3H); 7.23 (m, 2H); 7.08 (m, 2H); 6.81-6.87 (m, 2H); 6.51 (d, 1H); 3.87-3.7 (m, 2H, OCH2); 3.44-3.30 (m, 2H, OCH2); 1.88 (m, 1H, H-C); 1.71 (m, 1H, H-C); 1.62-1.42 (m) 2H, CH2); 1.39 (s, 9H, C(CH3)3); 1.29 (s, 9H, C(CH3)3); 1.10-1.33 (m, 4H, CH2); 1.07-0.83 (m, 12H, 4*CH3).
References:
US2007/265473,2007,A1 Location in patent:Page/Page column 12