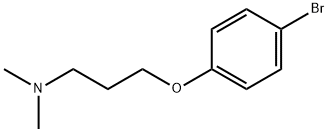
3-(4-Bromophenoxy)-N,N-dimethylpropylamine synthesis
- Product Name:3-(4-Bromophenoxy)-N,N-dimethylpropylamine
- CAS Number:76579-64-1
- Molecular formula:C11H16BrNO
- Molecular Weight:258.15
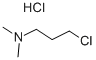
106-41-2
498 suppliers
$13.00/5g
3179-63-3
194 suppliers
$10.60/1gm:

76579-64-1
20 suppliers
inquiry
Yield:76579-64-1 56.3%
Reaction Conditions:
with di-tert-butyl-diazodicarboxylate;triphenylphosphine in dichloromethane at 0 - 30; for 3 h;Inert atmosphere;
Steps:
3-(4-Bromophenoxy)-NrV-dimeth l-propan-l-amine
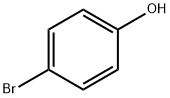
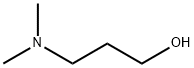
3-(4-Bromophenoxy)-NrV-dimeth l-propan-l-amine Di-tert-butyl azodicarboxylate (639 mg, 2.77 mmol) was added dropwise to a suspension of 4-bromophenol (400 mg, 2.31 mmol), 3-(dimethylamino)propan-l-ol (0.328 mL, 2.77 mmol) and triphenylphosphine (728 mg, 2.77 mmol) in DCM (3 mL) at 0°C then the mixture was allowed to warm to ambient temperature and stirred for 3 h. The reaction mixture was purified by ion exchange chromatography, using an SCX column and eluting with 1M NH3/MeOH. The desired material was further purified by FCC, elution gradient 0 to 10% MeOH in DCM, to afford the desired material as a colourless oil (336 mg, 56.3 %>). NMR Spectrum: NMR (500MHz, CDCb) δ 1.94 (2H, dq), 2.25 (6H, s), 2.4 - 2.47 (2H, m), 3.98 (2H, t), 6.74 - 6.82 (2H, m), 7.31 - 7.39 (2H, m). Mass Spectrum: m/z (ES+)[M+H]+ = 258.
References:
WO2017/76895,2017,A1 Location in patent:Page/Page column 47
7497-87-2
21 suppliers
$93.00/1g

124-40-3
545 suppliers
$18.00/100ml

76579-64-1
20 suppliers
inquiry