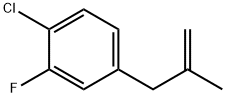
3-(4-Chloro-3-fluorophenyl)-2-methylprop-1-ene synthesis
- Product Name:3-(4-Chloro-3-fluorophenyl)-2-methylprop-1-ene
- CAS Number:787585-34-6
- Molecular formula:C10H10ClF
- Molecular Weight:184.64
170793-00-7
40 suppliers
$204.00/50ml

563-47-3
241 suppliers
$10.00/5g

787585-34-6
16 suppliers
$252.00/1g
Yield:-
Reaction Conditions:
copper(l) iodide in tetrahydrofuran at 0 - 20; for 1 h;
Steps:
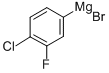
1-2.1 Step 1; 1-Chloro-2-fluoro-4-(2-methylallyl)benzene
Magnesium (2.43 g) and iodine (10 mg) were added to tetrahydrofuran (40 ml) and a solution of 4-bromo-1-chloro-2-fluorobenzene (21.0 g) in tetrahydrofuran (40 ml) was added dropwise, and the mixture was stirred at room temperature for 1 hr to give a Grignard's reagent. The reaction mixture was ice-cooled and copper iodide (1.90 g) was added. 3-Chloro-2-methyl-1-propene (14.8 ml) was added and the mixture was stirred at room temperature for 1 hr. The reaction mixture was ice-cooled and saturated aqueous ammonium chloride solution (10 ml) was added. The mixture was stirred at room temperature for 20 min and magnesium sulfate (40 g) was added. The reaction mixture was filtered and the filtrate was concentrated under reduced pressure. Hexane (100 ml) was added to the obtained residue and insoluble materials were filtered off. The resulting solution was concentrated under reduced pressure to give the title compound (16.9 g). 1H-NMR (300 MHz, δppm, CDCl3) 7.40-7.20(1H, m), 6.99(1H, dd, J=9.9, 1.8 Hz), 6.91(1H, d, J=8.1 Hz), 4.84(1H, s), 4.73(1H, s), 3.28(2H, s), 1.66(3H, s).
References:
US2005/32796,2005,A1 Location in patent:Page/Page column 41-42