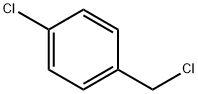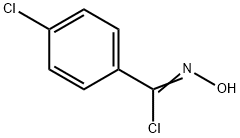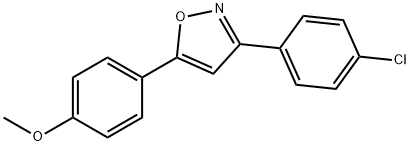
3-(4-Chlorophenyl)-5-(4-methoxyphenyl)isoxazole synthesis
- Product Name:3-(4-Chlorophenyl)-5-(4-methoxyphenyl)isoxazole
- CAS Number:24097-19-6
- Molecular formula:C16H12ClNO2
- Molecular Weight:285.73
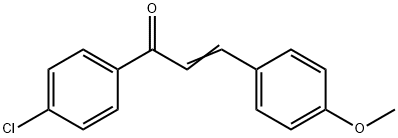
6552-63-2
46 suppliers
$21.00/250mg

24097-19-6
7 suppliers
inquiry
Yield:24097-19-6 77%
Reaction Conditions:
with hydroxylamine hydrochloride;sodium hydroxide in dimethyl sulfoxide at 100; for 8 h;
Steps:
Preparation of 3,5-disubstituted isoxazoles; general procedure
General procedure: A mixture of α,β-unsaturated ketone (0.5 mmol), HONH3Cl(1.0 mmol) and NaOH (2.0 mmol) in DMSO (5 mL) was stirredunder air at 100 oC for 8 h. After completion of the reaction, theresulting mixture was cooled to room temperature, diluted with ethylacetate and washed with saturated sodium carbonate solution. Theresulting organic phase was dried over anhydrous magnesium sulfateand concentrated under reduced pressure. The residue was isolatedby column chromatography using petroleum ether (boiling point:60-90 °C)/ethyl acetate (10:1) as eluent to give the pure product.
References:
Li, Zheng;Wen, Gong;Fu, Rugang;Yang, Jingya [Journal of Chemical Research,2016,vol. 40,# 10,p. 643 - 644]
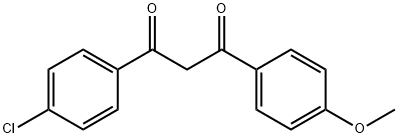
37975-19-2
5 suppliers
inquiry

24097-19-6
7 suppliers
inquiry
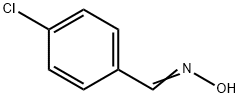
3848-36-0
62 suppliers
inquiry

24097-19-6
7 suppliers
inquiry