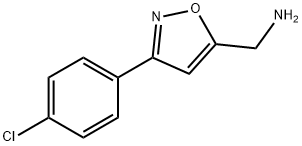
[3-(4-CHLOROPHENYL)-5-ISOXAZOLYL]METHANAMINE synthesis
- Product Name:[3-(4-CHLOROPHENYL)-5-ISOXAZOLYL]METHANAMINE
- CAS Number:66046-42-2
- Molecular formula:C10H9ClN2O
- Molecular Weight:208.64
![(NE)-N-[(4-chlorophenyl)methylidene]hydroxylamine](/CAS/GIF/3717-24-6.gif)
3717-24-6
5 suppliers
inquiry
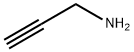
2450-71-7
328 suppliers
$14.00/1g
![[3-(4-CHLOROPHENYL)-5-ISOXAZOLYL]METHANAMINE](/CAS/GIF/66046-42-2.gif)
66046-42-2
24 suppliers
$65.00/100mg
Yield:66046-42-2 62%
Reaction Conditions:
Stage #1: 4-chlorobenzaldehyde oximewith N-chloro-succinimide in dichloromethane; for 0.333333 h;Heating / reflux;
Stage #2: Propargylaminewith triethylamine in dichloromethane; for 2 h;Heating / reflux;
Steps:
4; 13 Preparation of 3-p-chlorophenyl-5-aminomethyl-isoxazole.
Example 4 Preparation of 3-p-chlorophenyl-5-aminomethyl-isoxazole. 1.56 g (10mmol) of p-chlorobenzaldoxime was dissolved in 40ml of dried dichloromethane in a erlenmeyer flask equipped with a magnetic stirrer, and 1.7 g (12mmol) of N-chlorosuccinimide was added slowly. The reaction mixture was stirred until completely dissolved. The system was heated slightly for 20 min. Then, 0.56 g (10mmol) of propargyl amine was introduced and 1.2 g (12mmol) of triethylamine was added dropwise, which caused the emission of white smog. The reaction mixture was heated under reflux for 2 h, and then purified by column chromatography on silica gel using petroleum ether (b.p. 60-90°C) - ethyl acetate (v:v = 4:1) as eluant, to obtain 2.3 g of the product as yellow solid. The yield was 62%. 1H NMR (CDCl3), δ (ppm): 2.8 (s), 1H; 4.8 (s), 2H; 6.5 (s), 1H; 7.2-7.8 (m), 4H.
References:
EP1935892,2008,A1 Location in patent:Page/Page column 10
![3,5,7-Triaza-1-azoniatricyclo[3.3.1.13,7]decane, 1-[[3-(4-chlorophenyl)-5-isoxazolyl]methyl]-, bromide (1:1)](/CAS/20211123/GIF/66046-43-3.gif)
66046-43-3
0 suppliers
inquiry
![[3-(4-CHLOROPHENYL)-5-ISOXAZOLYL]METHANAMINE](/CAS/GIF/66046-42-2.gif)
66046-42-2
24 suppliers
$65.00/100mg
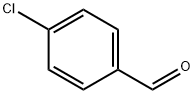
104-88-1
614 suppliers
$5.00/25g
![[3-(4-CHLOROPHENYL)-5-ISOXAZOLYL]METHANAMINE](/CAS/GIF/66046-42-2.gif)
66046-42-2
24 suppliers
$65.00/100mg
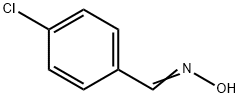
3848-36-0
61 suppliers
inquiry
![[3-(4-CHLOROPHENYL)-5-ISOXAZOLYL]METHANAMINE](/CAS/GIF/66046-42-2.gif)
66046-42-2
24 suppliers
$65.00/100mg
5300-92-5
18 suppliers
$195.00/250mg
![[3-(4-CHLOROPHENYL)-5-ISOXAZOLYL]METHANAMINE](/CAS/GIF/66046-42-2.gif)
66046-42-2
24 suppliers
$65.00/100mg