
3-(4-chlorophenyl)-N-methoxy-N-methylacrylamide synthesis
- Product Name:3-(4-chlorophenyl)-N-methoxy-N-methylacrylamide
- CAS Number:771557-40-5
- Molecular formula:C11H12ClNO2
- Molecular Weight:225.67
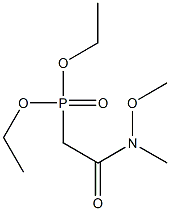
124931-12-0
89 suppliers
$21.00/250mg
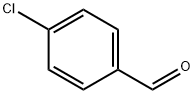
104-88-1
610 suppliers
$5.00/25g

771557-40-5
1 suppliers
inquiry
Yield:771557-40-5 74%
Reaction Conditions:
Stage #1: diethyl (N-methoxy-N-methylcarbamoylmethyl)phosphonatewith sodium hydride in tetrahydrofuran at 20;Horner-Wadsworth-Emmons Olefination;
Stage #2: 4-chlorobenzaldehyde in tetrahydrofuran at -78 - 20;Horner-Wadsworth-Emmons Olefination;
Steps:
HWE Reaction; General Procedure A
General procedure: Diethyl 2-[methoxy(methyl)amino]-2-oxoethylphosphonate (1; 1.2 equiv) was dissolved in anhyd THF and NaH (1.2 equiv) was added portionwise at r.t. until gas evolution ceased. The mixture was then cooled to -78 °C and the appropriate benzaldehyde derivative (1 equiv) dissolved in anhyd THF was added. The reaction mixture was stirred at -78 °C for 1 h, then allowed to warm to r.t. and stirred overnight. H2O (10 mL) was added, the layers were separated, and the organic layer was washed with sat. aq NaHCO3 (2 × 10 mL). The combined aqueous phases were extracted with EtOAc (3 × 15 mL). The combined organic phases were then washed with brine (2 × 10 mL) ,dried (MgSO4), and concentrated in vacuo. If necessary, the crude product was purified by flash column chromatography (FCC) using the indicated eluent.
References:
Glas, Carina;Bracher, Franz [Synthesis,2019,vol. 51,# 3,p. 757 - 768]
1615-02-7
319 suppliers
$7.00/5g

593-56-6
591 suppliers
$8.00/100dtn(0.1g)

771557-40-5
1 suppliers
inquiry

104-88-1
610 suppliers
$5.00/25g

771557-40-5
1 suppliers
inquiry

![Acetamide, 2-[[3,5-bis(trifluoromethyl)phenyl]sulfonyl]-N-methoxy-N-methyl-](/CAS/20210305/GIF/1048364-46-0.gif)