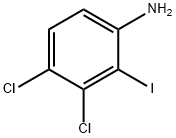
3,4-dichloro-2-iodobenzenamine synthesis
- Product Name:3,4-dichloro-2-iodobenzenamine
- CAS Number:835595-11-4
- Molecular formula:C6H4Cl2IN
- Molecular Weight:287.91
95-76-1
374 suppliers
$10.00/5g

835595-11-4
36 suppliers
$61.00/100mg
Yield:835595-11-4 15%
Reaction Conditions:
with hydrogen iodide;dihydrogen peroxide in water at 20;Darkness;
Steps:
2 Example 2: Synthesis of 3,4-dichloro-2-iodoaniline
[0092] To a stirred suspension of 3,4-dichloroaniline (20 g, 123 mmol), HI (48%>, 15.7 g, 123 mmol), H202 (30%, 8.3 g, 246 mmol) in H20 (62 mL) at r.t. The reaction mixture was stirred in dark at r.t for overnight. The supernatant was discarded, diluted with ethyl acetate: hexanes (1 : 10), quenched with saturates NaHS03, stirred for 1 h at ambient temperature, filtered the solids. The filtrate was washed with water, saturated aqueous NaHC03, dried (Na2S04), filtered and concentrated to get 1 :4 regioisomeric mixture of iodo derivative (minor isomer is required). It was recrystallized from cyclohexane to afford 1 : 1 mixture of regioisomers enriched in the filtrate. This mixture was further purified by flash column chromatography on silica gel, eluting with ethyl acetate :hexanes (0-2%) to get the required compound as a cream color solid (5.2 g, -15% yield). 1H NMR (400 MHz, CDC13) δ 7.22 (d, J= 8.6 Hz, 1 H), 6.60 (d, J= 8.6 Hz, 1 H), 4.32 (br, 2 H).
References:
WO2015/84842,2015,A1 Location in patent:Paragraph 0092