3,4-DICHLORO-5-PHENYL-2(5H)-FURANONE synthesis
- Product Name:3,4-DICHLORO-5-PHENYL-2(5H)-FURANONE
- CAS Number:72857-85-3
- Molecular formula:C10H6Cl2O2
- Molecular Weight:229.06
Yield:72857-85-3 76%
Reaction Conditions:
with aluminum (III) chloride at 20; for 2.5 h;
Steps:
34.A Step A: Preparation of 3,4-dichloro-5-phenylfuran-2(5H)-one
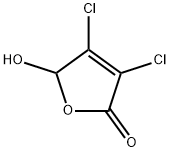
Example 34 1 -((3S.4R)-4-(3 ,4-difluorophenyl)- 1 -(2-methoxyethyl)pyrrolidin-3-yl)-3-(1 -methyl-6-oxo-3-phenyl- I ,6-dihydropyridazin-4-yl)urea1006211 Step A: Preparation of 3,4-dichloro-5-phenylfuran-2(5H)-one: To a suspension of A1CI3 (10.0 g, 75.0 mmol) in dry benzene (50 mL) was added 3,4-dichloro-5-hydroxyfuran- 2(5H)-one (8.45 g, 50.0 mmol) in portions over 5 minutes (immediate deep orange color). The mixture was stirred at ambient temperature for 2.5 hours and was poured into an ice-water slurry (300 mL). The mixture was stirred until ambient temperature was reached and extracted with Et20 (3X). The combined extracts were washed with H20 and saturated NaHCO3 (3X).The Et20 solution was dried over MgSO4 and filtered through a Si02 plug eluting with Et20. The filtrate was concentrated to provide the title compound a colorless syrup that solidified upon drying to a white solid (8.72 g, 76% yield). ‘H NMR (CDC13) 6 7.47-7.42 (m, 311), 7.3 1- 7.28 (m, 211), 5.85 (s, 1H) ppm.
References:
WO2014/78378,2014,A1 Location in patent:Paragraph 00621