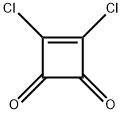
3,4-DICHLOROCYCLOBUT-3-ENE-1,2-DIONE synthesis
- Product Name:3,4-DICHLOROCYCLOBUT-3-ENE-1,2-DIONE
- CAS Number:2892-63-9
- Molecular formula:C4Cl2O2
- Molecular Weight:150.95
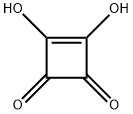
2892-51-5
385 suppliers
$5.00/1g

2892-63-9
30 suppliers
$90.00/100mg
Yield:2892-63-9 79%
Reaction Conditions:
with oxalyl dichloride;N,N-dimethyl-formamide in tetrachloromethane at 50; for 4 h;
Steps:
3,4-Dichlorocyclobut-3-ene-1,2-dione (squaric acid dichloride)
Experimental procedure taken from LUNELLI et al. [B. Lunelli, Tetrahedron Lett. 2007, 3595-3597]. Finely powdered squahc acid (1 1 .4 g, 0.10 mol) was suspended in anhydrous carbon tetrachloride (50 mL). After addition of anhydrous Λ/,/V-dimethylformamide (0.400 mL) and oxalyl chloride (18.0 mL, 0.200 mol), the reaction mixture was heated at 50 °C for four hours. After cooling to room temperature, the mixture was filtered, and the filtrate was evaporated under reduced pressure. The resulting oily residue crystallized in the refrigerator. Subsequently, the spontaneously precipitated, light yellow, easily sublimating crystals were separated, washed with petroleum ether and stored in the refrigerator. The product (Yield: 79%) was used without any further purification. Mp = 50 - 53 °C. (53 °C [B. Lunelli, Tetrahedron Lett. 2007, 3595-3597]).
References:
TECHNISCHE UNIVERSITAET BRAUNSCHWEIG;YISSUM RESEARCH DEVELOPMENT COMPANY OF THE HEBREW UNIVERSITY OF JERUSALEM LTD.;KUNICK, Conrad;LANDE, Hoang;GRUENEFELD, Johann;DZIKOWSKI, Ron;NASEREDDIN, Abed WO2017/8826, 2017, A1 Location in patent:Page/Page column 19-20

79-37-8
462 suppliers
$17.67/10gm:

2892-51-5
385 suppliers
$5.00/1g

2892-63-9
30 suppliers
$90.00/100mg

3200-96-2
7 suppliers
$57.00/250mg

2892-63-9
30 suppliers
$90.00/100mg