
(3,4-DICHLOROPHENYL)ACETALDEHYDE synthesis
- Product Name:(3,4-DICHLOROPHENYL)ACETALDEHYDE
- CAS Number:93467-56-2
- Molecular formula:C8H6Cl2O
- Molecular Weight:189.04
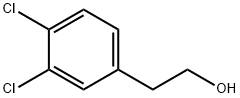
35364-79-5
127 suppliers
$14.00/1g

93467-56-2
24 suppliers
$231.00/250mg
Yield:93467-56-2 77%
Reaction Conditions:
with Dess-Martin periodane in dichloromethane at 20; for 1.5 h;
Steps:
8
[00125] A mixture of the above material (1.50 g, 7.85 mmol) and DMP (4.0 g, 9.4 mmol) in DCM (40 mL) was stirred at RT for 1.5 h, forming a white suspension. Hexanes (100 mL) was added, and the suspension was filtered through a celite cake. The filtrate was collected and concentrated to dryness under reduced pressure, and the residue was purified on silica gel by flash chromatography (EtOAc/hexanes, 1:6 to 1:4), affording 2-(3,4-dichlorophenyl)acetaldehyde as a pale green liquid (1.15 g, 77%). 1H NMR (400 MHz, CDCl3) δ 9.75 (t, J = 1.9 Hz, 1H), 7.44 (d, J = 8.2 Hz, 1H), 7.32 (d, J = 2.1 Hz, 1H), 7.05 (dd, J = 8.2, 2.1 Hz, 1H), 3.68 (d, J = 1.9 Hz, 2H).
References:
WO2021/224864,2021,A1 Location in patent:Paragraph 00125
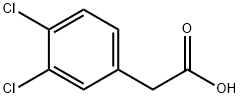
5807-30-7
281 suppliers
$13.00/5g

93467-56-2
24 suppliers
$231.00/250mg