
3',4'-(DIDECYLOXY)BENZALDEHYDE synthesis
- Product Name:3',4'-(DIDECYLOXY)BENZALDEHYDE
- CAS Number:118468-34-1
- Molecular formula:C27H46O3
- Molecular Weight:418.65
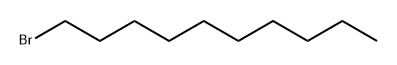
112-29-8
398 suppliers
$6.00/25g
584-08-7
1185 suppliers
$5.00/100g
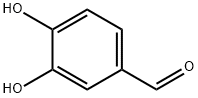
139-85-5
813 suppliers
$5.00/1g

118468-34-1
24 suppliers
$496.00/5g
Yield: 53%
Reaction Conditions:
in acetone
Steps:
8.A A.
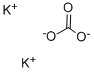
3,4-Dihydroxybenzaldehyde (10 g., 72.4 mmol, 1 equiv.) is dissolved in 150 mL of acetone. 1-Bromodecane (45.1 mL, 0.217 mol, 3 equiv.) is added, followed by K2 CO3 (10 g., 72.4 mmol, 1 equiv.), and the mixture heated to reflux for 24 hrs. At this time the reaction was incomplete, and another 10 grams of K2 CO3 was added and heating continued for 24 hours. The mixture is allowed to cool to room temperature, and is partitioned between ether and water. The aqueous layer is extracted thrice with ether, and the combined organic phases are washed with brine, dried (MgSO4), and evaporated. Upon evaporation, tan crystals formed, and these were collected. Further evaporation yielded another crop of crystals. A total of 16 grams of 3,4-didecyloxybenzaldehyde (38.3 mmol, 53%) was obtained (for an alternative synthesis of this compound see Strzelecka, H.; et al. Mol. Cryst. Liq. Cryst. 1988, 156 (Part A), 347): mp=59.5°-60.5° C.; UVmax (EtOH) 232 nm (ε=15472), 276 nm (ε=11079), 206 nm (ε=9806), 310 nm (ε=9273); 1 H NMR (300 MHz, CDCl3) δ 9.81 (s, 1H, CHO), 7.39 (dd, J=2, 8 Hz, 1H, ArH), 7.37 (d, J=2 Hz, 1H, ArH), 6.93 (d, J=8 Hz, 1H, ArH), 4.04 (m, 4H, RCH2 O), 1.83 (m, 4H, RCH2 CH2 O), 1.46 (m, 4H, decyl), 1.25 (m, 24H, decyl), 0.86 (m, 6H, 2*CH3); 13 C NMR (75 MHz, CDCl3) δ 190.98 (C=O), 154.68, 149.44, 129.87, 126.57, 111.76, 110.97, 69.13, 31.90, 29.57, 29.34, 29.07, 28.98, 25.97, 25.94, 22.68, 14.10; IR (KBr) 2956, 2922, 2850, 1688, 1674, 1596, 1586, 1510, 1278, 1134, 808 cm-1; MS (DCI) m/e 419 (MH+). Anal. Calcd for C27 H46 O3: C, 77.46; H, 11.07. Found: C, 77.19; H, 10.99. For 3,4-bis (3-methyl-2-butenyloxy)benzaldehyde: scale=6.75 mmol, yield=80%; chromatographed using EtOAc/hexane; UVmax (CHCl3) 280 nm (ε=11088), 312 nm (ε=9626), 240 nm (ε=7701); 1 H NMR (300 MHz, CDCl3) δ 9.80 (s, 1H, CHO), 7.38 (m, 2H, ArH), 6.93 (m, 1H, ArH), 5.47 (m, 2H, C=CH), 4.65 (d, J=6 Hz, 2H, RCH2 O), 4.61 (d, J=6 Hz, 2H, RCH2 O), 1.75 (s, 6H, 2*CH3), 1.72 (s, 6H, 2*CH3); 13 C NMR (75 MHz, CDCl3) δ 190.97 (C=O), 154.32, 149.11, 138.04, 137.98, 129.83, 126.51, 119.43, 119.28, 112.04, 111.11, 66.02, 65.93, 25.82, 25.79, 18.32, 18.29; IR (film) 2976, 2932, 2916, 1684, 1584, 1506, 1436, 1260, 1230, 1132, 1004 cm-1; MS (DCI) m/e 275 (MH+). Anal. Calcd for C17 H22 O3: C, 74.42; H, 8.08. Found: C, 74.41; H, 8.16.
References:
Bristol-Myers Squibb US5391817, 1995, A

112-29-8
398 suppliers
$6.00/25g

139-85-5
813 suppliers
$5.00/1g

118468-34-1
24 suppliers
$496.00/5g

2050-77-3
134 suppliers
$19.00/5g

139-85-5
813 suppliers
$5.00/1g

118468-34-1
24 suppliers
$496.00/5g