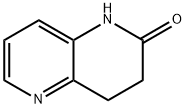
3,4-Dihydro-1,5-naphthyridin-2(1h)-one synthesis
- Product Name:3,4-Dihydro-1,5-naphthyridin-2(1h)-one
- CAS Number:943537-93-7
- Molecular formula:C8H8N2O
- Molecular Weight:148.16
![2-Pyridinepropanoic acid, 3-[[(1,1-dimethylethoxy)carbonyl]amino]-, ethyl ester](/CAS/20210305/GIF/1392407-48-5.gif)
1392407-48-5
0 suppliers
inquiry

943537-93-7
25 suppliers
$45.00/10mg
Yield:943537-93-7 74%
Reaction Conditions:
with trifluoroacetic acid in dichloromethane at 20;
Steps:
3,4-Dihydro-1H-[1,5]naphthyridin-2-one (27a):
10g 3-(3-tert-Butoxycarbonylamino-pyridin-2-yl)-propionic acid ethyl ester (34 mmol) was dissolved in 50 mL dichloromethane and added 50 mL TFA, and stirred over night at room temperature. The solvent was removed by evaporation, and ice was added to the residue, which was basicified (pH 9) with NH4OH. The mixture was evaporated and the residue taken up in 250 mL dichloromethane. The precipitated ammonium triflate was filtered off, and the product was recrystallized from ethylacetate. Yield = 3.7g (25 mmol, 74%)
References:
Sams, Anette Graven;Larsen, Krestian;Mikkelsen, Gitte Kobberoe;Hentzer, Morten;Christoffersen, Claus Tornby;Jensen, Klaus Gjervig;Frederiksen, Kristen;Bang-Andersen, Benny [Bioorganic and Medicinal Chemistry Letters,2012,vol. 22,# 15,p. 5134 - 5140] Location in patent:supporting information; experimental part

13993-61-8
95 suppliers
$21.00/250mg

943537-93-7
25 suppliers
$45.00/10mg
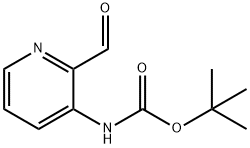
116026-99-4
85 suppliers
$98.00/100mg

943537-93-7
25 suppliers
$45.00/10mg