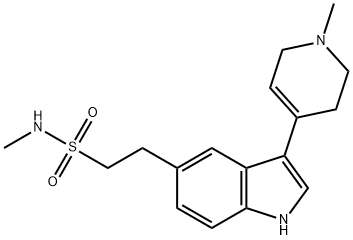
3,4-Dihydro Naratriptan (Naratriptan Impurity B) synthesis
- Product Name:3,4-Dihydro Naratriptan (Naratriptan Impurity B)
- CAS Number:121679-20-7
- Molecular formula:C17H23N3O2S
- Molecular Weight:333.45
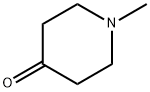
1445-73-4
463 suppliers
$15.00/25g
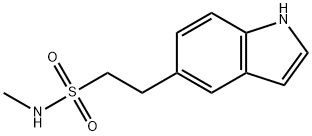
98623-50-8
82 suppliers
inquiry

121679-20-7
23 suppliers
$43.00/1mg
Yield:121679-20-7 90%
Reaction Conditions:
Stage #1: 1-Methyl-4-piperidone;2-(1H-indol-5-yl)ethanesulfonic acidmethylamidewith potassium hydroxide in ethanol;Reflux;
Stage #2: with water in ethanol at 10 - 20;Product distribution / selectivity;
Steps:
5.2
Method 2: A mixture of the compound (VII) (51 g, 21.42mmol), N-Methyl-4- pipperidone (60.61g, 53.56mmol) and potassium hydroxide (30g, 53.57mmol) in ethanol(0.357 lit) was refluxed for 6-8 hrs. The obtained reaction mixture was cooled to 20°C and quenched slowly in 1.5 lit chilled water (at 10-15°C). The precipitated solid was stirred for one hour. The obtained residue was washed with water till pH 7 to 8. and then wash with chilled ethanol to get compound (VIII) (55g).The compound (VIII) (55 g) in methanol (1.65 L) was refluxed for one hour till to get clear solution. The obtained solution was filtered through hyflow. The solvent was partially distilled out. The remaining mixture was cooled to 10°C. The solid obtained was filtered and washed with chilled methanol and dried. [Yield: 49.5g, 90%].
References:
WO2009/118753,2009,A2 Location in patent:Page/Page column 27

98623-16-6
61 suppliers
$53.00/25mg

121679-20-7
23 suppliers
$43.00/1mg
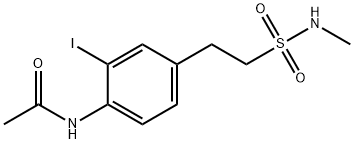
1268265-90-2
1 suppliers
inquiry

121679-20-7
23 suppliers
$43.00/1mg
![Acetamide, N-[4-[2-[(methylamino)sulfonyl]ethyl]-2-[2-(trimethylsilyl)ethynyl]phenyl]-](/CAS/20210305/GIF/1268265-95-7.gif)
1268265-95-7
1 suppliers
inquiry

121679-20-7
23 suppliers
$43.00/1mg
1200070-42-3
4 suppliers
inquiry

121679-20-7
23 suppliers
$43.00/1mg