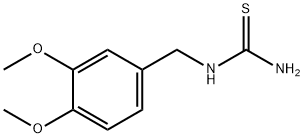
[(3,4-dimethoxyphenyl)methyl]thiourea synthesis
- Product Name:[(3,4-dimethoxyphenyl)methyl]thiourea
- CAS Number:14596-51-1
- Molecular formula:C10H14N2O2S
- Molecular Weight:226.3

14596-50-0
27 suppliers
$70.00/1g
![[(3,4-dimethoxyphenyl)methyl]thiourea](/CAS/20200611/GIF/14596-51-1.gif)
14596-51-1
6 suppliers
inquiry
Yield:14596-51-1 87.3%
Reaction Conditions:
with ammonia in tetrahydrofuran;water; for 0.75 h;
Steps:
15.A Example 15; 3-(3,4-Dimethoxy-benzyl)-8-(1-methyl-ethyl)-hypoxanthine; A. N-(3,4-Dimethoxy-benzyl)-thiourea
28.54 g/22.65 ml (375 MM) of carbon disulfide were added with cooling to a stirred solution of 10 g (250 MM) of NaOH in 35 ml of water, within 25 min a solution 39.1 ml (250 MM) of 95% veratrylamine in 20 ml of water were added and the mixture was heated to 75 °C within 20 min and the solution was kept for 1 hr at 75 to 80°C. After cooling to 45°C 23.8 ml (250 MM) of ethyl chloroformate were added slowly.. Within 30 min 8 ml of 2n NaOH were added dropwise, the PH elevated from 6 to 8 and the formation of COS started.. After about 1 hr the gas formation ceded and after a further 30 min 150 ml of ether were added to dilute the lower mustard oil phase.. The now upper ether phase was collected and washed with half saturated NaCl solution. 58.8 g (112%) of isothiocyanate which was dissolved in 60 ml of THF and treated with 30.2 ml of 32% aqueous ammonia solution (exothermic).. After 45 min the solvents were removed in vacuo and the residue suspended in 100 ml of water.. The solid was collected (56.2 g), suspended in 130 ml of hot acetone and the solid collected again at 5°C: 49.37 g (87.3%) of thiourea with mp 192-193°C.
References:
EP1202628,2004,B1 Location in patent:Page 17