
3,4-DIMETHYLTHIOANISOLE synthesis
- Product Name:3,4-DIMETHYLTHIOANISOLE
- CAS Number:65398-69-8
- Molecular formula:C9H12S
- Molecular Weight:152.26
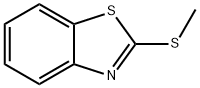
615-22-5
183 suppliers
$10.00/5g
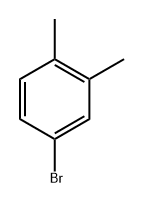
583-71-1
148 suppliers
$13.43/1gm:

65398-69-8
15 suppliers
$45.00/100mg
Yield:65398-69-8 84%
Reaction Conditions:
with 1,1'-bis(diphenylphosphanyl)ferrocene;Potassium phosphate, dibasic;[1,2-bis(diphenylphosphino)ethane]dichloronickel(II);palladium diacetate;zinc powder in N,N-dimethyl acetamide at 100; for 12 h;Inert atmosphere;Molecular sieve;
Steps:
6 Example 6
1.0 equiv of benzothiazole methyl sulfide (0.2 mmol, 36.2 mg), 1.0 equiv of 4-bromo-1,2-dimethylbenzene (0.2 mmol, 36.8 mg), Pd(OAc) (0.02 mmol, 4.5mg) and Ni(dppe)Cl2 (0.02mmol, 10.6mg), 1-1'-bis(diphenylphosphonium)ferrocene (0.02mmol, 11.1mg), 2.0 equiv of dipotassium hydrogen phosphate (0.4mmol) , 69.7 mg) and 2.5 equivalents of zinc powder (0.5 mmol, 32.5 mg) were put into the reaction flask. After three nitrogen purges, dry dimethylacetamide (2.0 mL) was added. After stirring at 100°C for 12 hours, saturated sodium chloride solution was added to the reaction system to quench the reaction, then the organic phase was extracted three times with dichloromethane, the organic phase was dewatered by anhydrous sodium sulfate, then the organic solvent was removed under reduced pressure, and the organic phase was removed by rapid Column machine gave a colorless liquid (25.5 mg, 84%).
References:
CN114149405,2022,A Location in patent:Paragraph 0072-0074