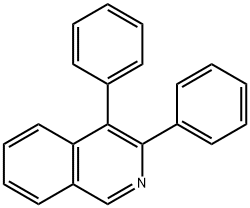
3,4-DIPHENYLISOQUINOLINE synthesis
- Product Name:3,4-DIPHENYLISOQUINOLINE
- CAS Number:52839-45-9
- Molecular formula:C21H15N
- Molecular Weight:281.35
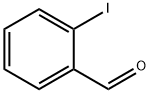
26260-02-6
242 suppliers
$8.00/250mg
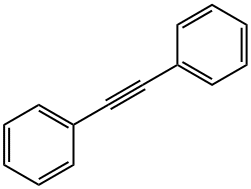
501-65-5
215 suppliers
$16.00/5g

52839-45-9
17 suppliers
inquiry
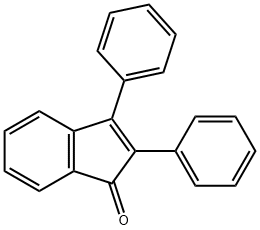
1801-42-9
38 suppliers
$390.00/10g
Yield:52839-45-9 91% ,1801-42-9 3%
Reaction Conditions:
Stage #1: 2-iodobenzaldehydewith tert-butylamine in N,N-dimethyl-formamide;Inert atmosphere;
Stage #2: diphenyl acetylenewith [Pd(η(5)-C5H5)Fe(η(5)-C5H3-C(CH3)=N-C6H4-4-CH3)Cl(P(C6H5)3)];sodium carbonate;lithium chloride in N,N-dimethyl-formamide at 100; for 24 h;Inert atmosphere;
Steps:
4.2. General procedure for synthesis of isoquinolines
General procedure: To a solution of o-halobenzaldehyde (0.25 mmol) in DMF (1 mL), tert-butyl amine (0.75 mmol) was added. The resulting mixture was stirred under a nitrogen atmosphere at room temperature for 12 h (or 100 °C for 4 h). After the haloaldehyde was consumed completely (monitored by TLC), alkynes (0.50 mmol), Na2CO3 (0.50 mmol), LiCl (0.25 mmol), palladacycle (1 mol %), and DMF (1 mL) were added to the mixture. Then the vial was placed in a preheated oil bath and heated at 100 °C under stirring for 24 h. After the reaction was complete (monitored by TLC), the mixture was diluted with CH2Cl2 (10 mL), filtered through a pad of Celite, and extracted with CH2Cl2. The combined organic phase was dried over anhydrous Na2SO4, filtered, and evaporated under reduced pressure. The residue was purified by flash chromatography on silica gel (ethyl acetate/hexane) to afford the pure product.
References:
Yang, Fan;Zhang, Junli;Wu, Yangjie [Tetrahedron,2011,vol. 67,# 16,p. 2969 - 2973] Location in patent:experimental part

501-65-5
215 suppliers
$16.00/5g
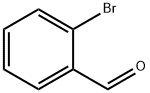
6630-33-7
411 suppliers
$9.00/10g

52839-45-9
17 suppliers
inquiry

1801-42-9
38 suppliers
$390.00/10g
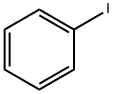
591-50-4
483 suppliers
$10.00/1g
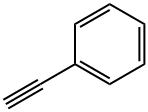
536-74-3
305 suppliers
$10.00/1g
![2-Propanamine, N-[(2-iodophenyl)methylene]-2-methyl-](/CAS/20210305/GIF/212505-57-2.gif)
212505-57-2
0 suppliers
inquiry

52839-45-9
17 suppliers
inquiry
![2-Propanamine, N-[(2-bromophenyl)methylene]-2-methyl-](/CAS/20210305/GIF/95547-11-8.gif)
