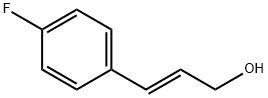
3-(4-Fluoro-phenyl)-prop-2-en-1-ol synthesis
- Product Name:3-(4-Fluoro-phenyl)-prop-2-en-1-ol
- CAS Number:124980-95-6
- Molecular formula:C9H9FO
- Molecular Weight:152.17
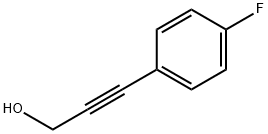
80151-28-6
42 suppliers
inquiry

124980-95-6
22 suppliers
inquiry
Yield:124980-95-6 95%
Reaction Conditions:
with lithium aluminium tetrahydride in tetrahydrofuran at 0 - 23; for 6 h;stereoselective reaction;
Steps:
4.4.1. (E)-(3-Iodoprop-1-en-1-yl)benzene (1a)
General procedure: A 50 mL oven-dried flask was charged with LiAlH4 (190 mg,5 mmol) and dry THF (15 mL). The mixture was cooled to 0 C, followed by a slow addition of 11a (660 mg, 5 mmol, in 5 mL THF). The mixture was stirred at room temperature until the reaction was complete (monitored by 1H NMR). The mixture was then cooled to 0 C and carefully quenched by H2O (1.2 mL), 15% NaOH (1.2 mL)and H2O (1.2 mL) to afford a suspension, which was filtered through Celite and washed with EtOAc (30 mL x 3). The combined solution was concentrated and purified by column chromatography on silica gel (Hexane/EtOAc 3:1, v/v) to afford (E)-3-phenylprop-2-en-1-ol (12a, 636 mg, 95% yield) as a colorless oil.
References:
Xu, Bin;Gartman, Jackson A.;Tambar, Uttam K. [Tetrahedron,2017,vol. 73,# 29,p. 4150 - 4159]