
3-(4-ISOPROPYLPHENYL)-2-PROPEN-1-OL synthesis
- Product Name:3-(4-ISOPROPYLPHENYL)-2-PROPEN-1-OL
- CAS Number:274907-08-3
- Molecular formula:C12H16O
- Molecular Weight:176.25
![2-Propenoic acid, 3-[4-(1-methylethyl)phenyl]-, methyl ester, (E)-](/CAS/20180527/GIF/69358-86-7.gif)
69358-86-7
1 suppliers
inquiry

274907-08-3
9 suppliers
$244.00/500mg
Yield:274907-08-3 81%
Reaction Conditions:
with lithium borohydride in tetrahydrofuran;diethyl ether at 0 - 20; for 16 h;
Steps:
4.3. Reduction of esters 2a-e
General procedure: General procedure: A 2 M solution of LiBH4 in THF (16.5 mL) wasadded dropwise to a solution of ester 2a-e (0.0165 mol) in anhydrousdiethyl ether (50 mL) at 0 C. The mixture was stirred atroom temperature for 16 h, poured onto ice and diluted HCl wasadded carefully to quench the excess of hydride reagent. The aqueousand ethereal layers were separated. The aqueous layer was extractedthree times with diethyl ether (15 mL). The combinedetheral solutions were washed with saturated brine, H2O, driedover MgSO4 and concentrated in vacuo to give crude alcohols 3a-e. After purification by column chromatography with hexane-ethylacetate (3:1) as eluent, pure alcohols 3a-e were obtained as colorlessoils. Their physical and spectral data were the same as reportedin literature [18].
References:
Wzorek, Alicja;Gawdzik, Barbara;Gladkowski, Witold;Urbaniak, Mariusz;Barańska, Anita;Malińska, Maura;Wo?niak, Krzysztof;Kempinska, Katarzyna;Wietrzyk, Joanna [Journal of Molecular Structure,2013,vol. 1047,p. 160 - 168]
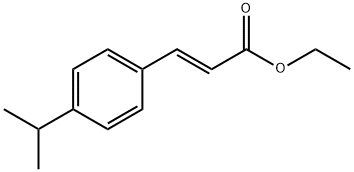
128408-02-6
5 suppliers
inquiry

274907-08-3
9 suppliers
$244.00/500mg
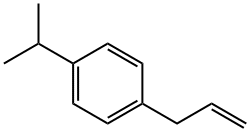
3897-64-1
10 suppliers
$252.00/1g

274907-08-3
9 suppliers
$244.00/500mg
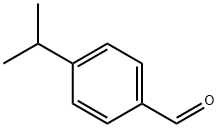
122-03-2
327 suppliers
$6.00/25g

274907-08-3
9 suppliers
$244.00/500mg