
3-(4-METHYLPHENOXY)BENZYLAMINE HYDROCHLORIDE synthesis
- Product Name:3-(4-METHYLPHENOXY)BENZYLAMINE HYDROCHLORIDE
- CAS Number:154108-16-4
- Molecular formula:C14H15NO
- Molecular Weight:213.27
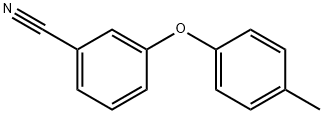
253679-51-5
0 suppliers
inquiry

154108-16-4
19 suppliers
$140.00/100mg
Yield:-
Reaction Conditions:
Stage #1: 3-(4-methylphenoxy)benzonitrilewith lithium aluminium tetrahydride in tetrahydrofuran at 0 - 20;
Stage #2: with water;sodium hydroxide in tetrahydrofuran;
Steps:
66
A solution produced by dissolving 8.0 g of 3-(4-methylphenoxy)benzonitrile in 5 ml ofTHF was added dropwise to a mixture solution of 1.8 g of lithium aluminum hydride and 50 ml of THF at 0°C. The obtained mixture was stirred at a room temperature for 4 hours. Thereafter, water, 15% sodium hydroxide aqueous solution and water were successively added to the reaction mixture, and the obtained mixture was then left overnight. Thereafter, the reaction mixture was filtrated through Celite (registered trade mark), and the filtrate was then concentrated under reduced pressure. MTBE and potassium carbonate were added to the residue. The resultant was filtrated through Celite (registered trade mark), and the filtrate was then concentrated under reduced pressure so as to obtain 7.0 g of crude 3-(4-methylphenoxy)benzylamine.3-(4-methylphenoxy)benzylamine [Show Image] 1H-NMR (CDCl3) δ: 1.40 (2H, br s), 2.33 (3H, s), 3.82 (2H, s), 6.82-6.87 (1H, m), 6.89-6.95 (3H, m), 6.99-7.03 (1H, m), 7.11-7.15 (2H, m), 7.24-7.28 (1H, m).
References:
EP2248423,2010,A1 Location in patent:Page/Page column 263