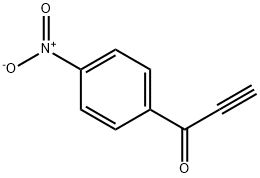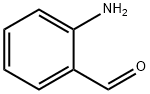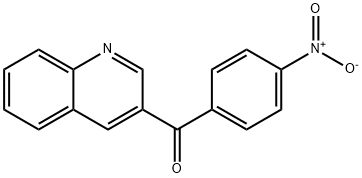
3-(4-Nitrobenzoyl)quinoline synthesis
- Product Name:3-(4-Nitrobenzoyl)quinoline
- CAS Number:1187166-16-0
- Molecular formula:C16H10N2O3
- Molecular Weight:278.26
Yield:1187166-16-0 81%
Reaction Conditions:
with iron(III) chloride hexahydrate in 1,4-dioxane at 80; for 4 h;Michael addition-cyclization;
Steps:
A typical procedure for the FeCl3·6H2O catalyzed one-pot synthesis of 3-carbonyl quinolines from the reactions of ynones with 2-aminobenzaldehydes:
A solution of 1-phenylprop-2-yn-1-one (2a) (32.5 mg, 0.25 mmol) in 1,4-dioxane (2 ml) was added 2-aminobenzaldehyde (1a) (61 mg, 0.5 mmol) and FeCl3·6H2O (13.4 mg, 20 mol %), and the resulted reaction mixture was stirred at 80 oC for 4 h. After cooling to room temperature, the solvent was evaporated under vacuo and the residue was purified by chromatography on silica gel. A yellow solid of product (49 mg, 84%) 3a was obtained.
References:
Li, Hongfeng;Xu, Xiaolei;Yang, Jingyu;Xie, Xin;Huang, He;Li, Yanzhong [Tetrahedron Letters,2011,vol. 52,# 4,p. 530 - 533] Location in patent:supporting information; experimental part
271-58-9
109 suppliers
$13.00/250mg
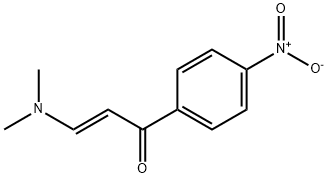
78089-99-3
24 suppliers
$45.00/100mg

1187166-16-0
7 suppliers
inquiry

90312-02-0
9 suppliers
inquiry

1187166-16-0
7 suppliers
inquiry
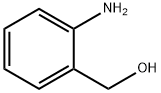
5344-90-1
325 suppliers
$6.00/5g

1187166-16-0
7 suppliers
inquiry
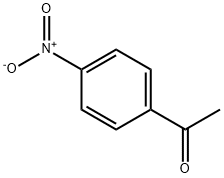
100-19-6
304 suppliers
$5.00/5g

1187166-16-0
7 suppliers
inquiry