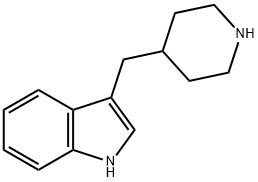
3-(4-Piperidylmethyl)-1H-indole synthesis
- Product Name:3-(4-Piperidylmethyl)-1H-indole
- CAS Number:3515-49-9
- Molecular formula:C14H18N2
- Molecular Weight:214.31
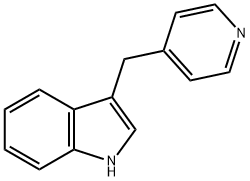
5275-07-0
12 suppliers
$599.00/1g

3515-49-9
21 suppliers
inquiry
Yield:3515-49-9 73%
Reaction Conditions:
Stage #1: 3-<(4-pyridyl)methyl>-1H-indolewith hydrogen;acetic acid;platinum(IV) oxide at 20; under 1551.49 Torr; for 2 h;
Stage #2: with sodium carbonate in water;ethyl acetate;
Steps:
8
3-(Pyridin-4-ylmethyl)-1H-indole (5.00 g, 24.0 mmol) and acetic acid (25 mL) were added to platinum(IV) oxide (1.00 g, 4.40 mmol, Johnson Matthey) in a 250 mL stainless steel pressure bottle and stirred for about 2 hr under about 30 psi hydrogen at ambient temperature. The mixture was filtered and the filtrate was concentrated under reduced pressure. The residue was dissolved with EtOAc and then washed with saturated aqueous Na2CO3. The organic layer became turbid. A small amount of MeOH was added to form a clear solution. The organic layer was washed with 2 N aqueous NaOH and brine, dried over MgSO4, filtered, and concentrated under reduced pressure. The resulting solid was triturated with Et2O and then dried to give 3-(piperidin-4-ylmethyl)-1H-indole (4.35 g, 73% yield): LC/MS (Table 1, Method a) Rt=1.51 min; MS m/z: 215.12 (M+H)+.
References:
US2011/152243,2011,A1 Location in patent:Page/Page column 41-42

63845-36-3
0 suppliers
inquiry

3515-49-9
21 suppliers
inquiry

51626-60-9
1 suppliers
inquiry

3515-49-9
21 suppliers
inquiry
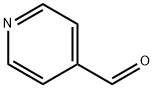
872-85-5
484 suppliers
$10.00/1g

3515-49-9
21 suppliers
inquiry
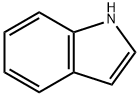
120-72-9
628 suppliers
$6.00/25g

3515-49-9
21 suppliers
inquiry