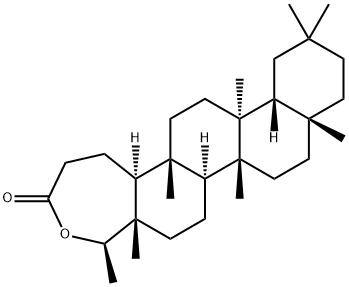
3,4-Seco-3,4-epoxyfriedelane-3-one synthesis
- Product Name:3,4-Seco-3,4-epoxyfriedelane-3-one
- CAS Number:29621-75-8
- Molecular formula:C30H50O2
- Molecular Weight:442.72
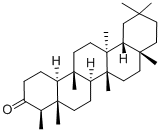
559-74-0
144 suppliers
$70.00/10mg

29621-75-8
13 suppliers
inquiry
Yield:29621-75-8 87%
Reaction Conditions:
with 3-chloro-benzenecarboperoxoic acid in chloroform; for 4 h;Reflux;Baeyer-Villiger Ketone Oxidation;
Steps:
General procedure for the synthesis of lactones 5 and 6
General procedure: A round-bottom flask was charged with compound 1 (165.3 mg, 0.39 mmol) or 2 (440.0 mg, 1.0 mmol) dissolved in chlorofom and m-chloroperbenzoic acid (m-CPBA) (1.43 mmol for 1 and 3.77 mmol for 2). The reaction mixture was stirred for 4 h under reflux, until TLC analysis revealed a total consumption of the starting material. Ice and a satured NaHCO3 solution were added to the flask until pH 7. The mixture was further extracted with chloroform (4 x 30 mL) and dried in a rotavap. Purifications were then performed with CC.
References:
Aguilar, Mariana G.;Duarte, Lucienir P.;Evangelista, Fernanda C. G.;Sabino, Adriano P.;Sousa, Grasiely F.;Vieira Filho, Sidney A. [Natural Product Research,2020,vol. 34,# 6,p. 810 - 815] Location in patent:supporting information
![Silane, trimethyl[[(4β,5β,8α,9β,10α,13α,14β)-5,9,13-trimethyl-24,25,26-trinorolean-2-en-3-yl]oxy]- (9CI)](/CAS/20210305/GIF/161739-59-9.gif)
161739-59-9
0 suppliers
inquiry

29621-75-8
13 suppliers
inquiry

19865-96-4
0 suppliers
inquiry

29621-75-8
13 suppliers
inquiry