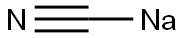3-(5-CHLORO-2-METHOXY-PHENYL)-3-OXO-PROPIONITRILE synthesis
- Product Name:3-(5-CHLORO-2-METHOXY-PHENYL)-3-OXO-PROPIONITRILE
- CAS Number:69316-10-5
- Molecular formula:C10H8ClNO2
- Molecular Weight:209.63
33924-48-0
151 suppliers
$43.39/25g
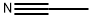
75-05-8
969 suppliers
$10.00/10g

69316-10-5
13 suppliers
inquiry
Yield:69316-10-5 94%
Reaction Conditions:
Stage #1: acetonitrilewith n-butyllithium;diisopropylamine in tetrahydrofuran;hexane at -78; for 0.5 h;Inert atmosphere;
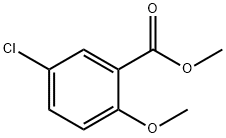
Stage #2: methyl 5-chloro-2-methoxybenzoate in tetrahydrofuran;hexane at -78; for 0.666667 h;
Steps:
xviii.a 3-(5-Chloro-2-methoxyphenyl)-3-oxopropanenitrile I37
To a solution of diisopropylamine (3.29 g, 32.5 mmol) in anhydrous THF (100 mL) at -78 °C under N2 was added n-butyllithium (2.5 M solution in hexanes, 13.0 mL, 32.5 mmol) dropwise and the mixture was stirred at -78 °C for 1 h. A solution of acetonitrile (1.33 g, 32.5 mmol) in anhydrous THF (20 mL) was then added dropwise and stirring was continued at -78 °C for 30 min. A solution of methyl 5-chloro-2-methoxybenzoate (5.0 g, 25.0 mmol) in anhydrous THF (10 mL) was then added and the mixture was stirred at -78 °C for 40 min. The reaction was quenched at -78 °C with 1 M HCI and the mixture was extracted with EtOAc (100 mL x 3). The combined organic extracts were washed with brine, dried over anhydrous Na2S04, filtered and concentrated to give the title compound I37 (4.9 g, 94%) as an orange solid, which was used in the next step without further purification. LCMS-A (ES-API): Rt2.10 min; m/z 209.9 [M+H]+, 231.9 [M+Na]+.
References:
WO2020/2587,2020,A1 Location in patent:Page/Page column 71
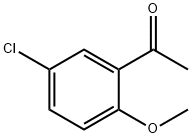
6342-64-9
100 suppliers
$15.00/1g

69316-10-5
13 suppliers
inquiry