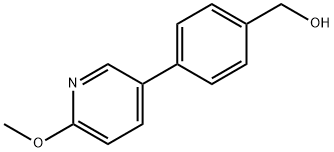
3-(5-Cyanopyridin-3-yl)benzyl alcohol synthesis
- Product Name:3-(5-Cyanopyridin-3-yl)benzyl alcohol
- CAS Number:328125-42-4
- Molecular formula:C13H13NO2
- Molecular Weight:215.25
59016-93-2
320 suppliers
$5.00/1g

328125-42-4
4 suppliers
inquiry
Yield:-
Reaction Conditions:
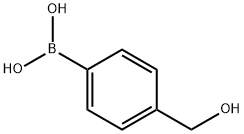
Stage #1: p-hydroxymethylphenylboronic acidwith potassium carbonate in 1,2-dimethoxyethane;water; for 0.333333 h;
Stage #2: tetrakis(triphenylphosphine) palladium(0) in 1,2-dimethoxyethane;water at 80; for 16 h;
Steps:
2a.1
To (4-hydroxymethylphenyl)boronic acid (Combi-B locks; 10.0g, 65.8mmol) in DME/H2O (60mL, 2: 1 ) was added 5-bromo-2-methoxypyridine (Combi-Blocks; 13.6g, 72.4mmol) and potassium carbonate (45.5g, 328.9mmol). The reaction was degassed with N2 for 20 minutes. Tetrakis(triphenylphosphine)palladium(0) (0.760g, 0.66mmol) was added and the reaction was further degassed for 15 minutes. The reaction was then heated to 80°C for 16 hours under N2. LCMS confirmed the formation of the product. The reaction was partitioned between H2O and EtOAc and the aqueous layer was extracted twice with EtOAc. The combined organic layers were dried over MgSO4, filtered, concentrated, and triturated with EtOAc to give the desired product.
References:
WO2008/67566,2008,A1 Location in patent:Page/Page column 92