
3,5-dichloro-4-hydroxybenzophenone synthesis
- Product Name:3,5-dichloro-4-hydroxybenzophenone
- CAS Number:34183-06-7
- Molecular formula:C13H8Cl2O2
- Molecular Weight:267.11
? without solvent at 154° for 2.5 h (71%);
? in chlorobenzene at 140–150° for 20 min;
? in nitrobenzene at 75° for 24 h.

38932-58-0
2 suppliers
inquiry

34183-06-7
6 suppliers
inquiry
Yield:34183-06-7 61%
Reaction Conditions:
with oxygen;cobalt(II) diacetate tetrahydrate;sodium hydroxide in ethylene glycol at 100; under 760.051 Torr; for 18 h;
Steps:
General procedure for preparation of 1
General procedure: A mixture of substrate 1 (2.0 mmol), Co(OAc)24H2O (40 mg, 0.16 mmol) andNaOH (160 mg, 4.0 mmol) in EG (9.0 ml) was stirred with molecular oxygen(1 atm) being bubbled, under 100 C for specified time. After completion ofreaction, diluted hydrochloric acid (15 ml, 2 %) and chloroform (15 ml) weresuccessively added to the reaction mixture. The organic layer was separated, and theaqueous phase was extracted with chloroform (15 ml 9 2). The combined organicphase was dried over anhydrous sodium sulfate and concentrated to give a residue,which was purified by column chromatography on silica gel (eluents: petroleumether:ethyl acetate = 10:1) providing the desired products 2.
References:
Huang, Jian-Gang;Guo, Ying;Jiang, Jian-An;Liu, Hong-Wei;Ji, Ya-Fei [Research on Chemical Intermediates,2015,vol. 41,# 10,p. 7115 - 7124]
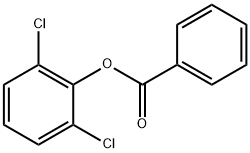
107931-00-0
0 suppliers
inquiry

34183-06-7
6 suppliers
inquiry

87-65-0
400 suppliers
$10.00/10g

34183-06-7
6 suppliers
inquiry

98-88-4
575 suppliers
$10.00/5g

34183-06-7
6 suppliers
inquiry
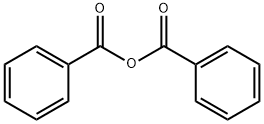
93-97-0
366 suppliers
$6.00/25g

34183-06-7
6 suppliers
inquiry