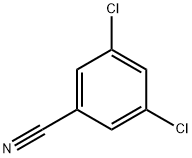
3,5-Dichlorobenzonitrile synthesis
- Product Name:3,5-Dichlorobenzonitrile
- CAS Number:6575-00-4
- Molecular formula:C7H3Cl2N
- Molecular Weight:172.01
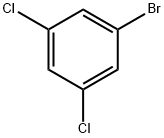
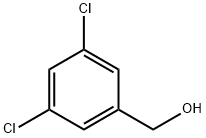
19752-55-7
368 suppliers
$8.00/10g

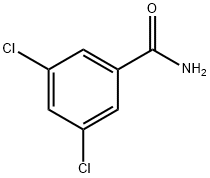
6575-00-4
266 suppliers
$6.00/1g
Yield:6575-00-4 72%
Reaction Conditions:
Stage #1:1-bromo-3,5-dichlorobenzene with isopropylmagnesium chloride;lithium chloride in tetrahydrofuran at -15; for 0.25 h;
Stage #2: with N,N-dimethyl-formamide in tetrahydrofuran at 0; for 2 h;
Stage #3: with ammonia;iodine in tetrahydrofuran;water at 20; for 2 h;
Steps:
3.28 Typical experimental procedure for conversion of aromaticbromides into aromatic nitriles with iPrMgCl, DMF, I2,and aq NH3
General procedure: To a flask containing dried LiCl (0.35 g, 8.24 mmol) was added iPrMgCl (2 M in THF, 4.1 mL) and THF (5 mL) at 15° C. After beingstirred for 15 min, 3-bromo-1-benzonitrile (1.46 g, 8.03 mmol) inTHF (1 mL) was added to the reaction mixture and the obtainedmixture was stirred for 15 min. Then, DMF (1.3 mL, 12 mmol) wasadded at 0° C and the mixture was stirred for 2 h. Then, aq NH3 (7 mL, 28-30%) and I2 (4.06 g, 16 mmol) were added to the reaction mixture. After being stirred for 2 h at room temperature, the reactionmixture was poured into satd aq Na2SO3 solution and was extracted with CHCl3 (3∗30 mL). The organic layer was dried over Na2SO4 and filtered. After removal of the solvent, the residue waspurified by short column chromatography on silica gel (eluent:hexane/ethyl acetate=9:1, v/v) to provide pure 1,3-dicyanobenzene (0.73 g) in 71% yield. Most nitriles mentioned in this work are commercially availableand were identified by comparison with the authentic samples.
References:
Ishii, Genki;Harigae, Ryo;Moriyama, Katsuhiko;Togo, Hideo [Tetrahedron,2013,vol. 69,# 5,p. 1462 - 1469]
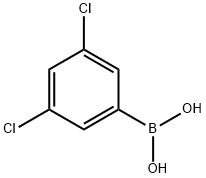
626-43-7
326 suppliers
$10.00/1g

6575-00-4
266 suppliers
$6.00/1g