
3,5-Dichloropyridine-2-carbonyl chloride synthesis
- Product Name:3,5-Dichloropyridine-2-carbonyl chloride
- CAS Number:70151-22-3
- Molecular formula:C6H2Cl3NO
- Molecular Weight:210.45
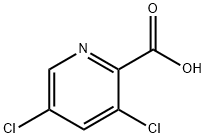
81719-53-1
144 suppliers
$7.00/1g

70151-22-3
12 suppliers
$236.81/1gm:
Yield:70151-22-3 100%
Reaction Conditions:
with oxalyl dichloride;N,N-dimethyl-formamide in dichloromethane at 0 - 20; for 2 h;
Steps:
3,5-dichloro-2-(chloromethyl)pyridine; [00376] To a 0 °C solution of 5-chloropicolinic acid (5.00 g, 26.0 mmol) and N,N- dimethylformamide (1 drop) in dichloromethane (20 mL) was added oxalyl chloride (3.28 g, 26.0 mmol) dropwise, after which the reaction mixture was allowed to warm up to room temperature and stirred at that temperature for two hours. The reaction was then cooled again to 0 °C, after which methanol (10 mL) was added dropwise to the reaction mixture, and the reaction was allowed to stir at room temperature for one hour where it was shown as complete by LCMS analysis. The reaction mixture was washed with saturated sodium bicarbonate solution, dried (magnesium sulfate), filtered and concentrated to afford methyl 3,5-dichloropyridine-2-carboxylate (5.36 g, 26.0 mmol, 100% yield ) as a white solid.[00377] To a 0 °C solution of methyl 3,5-dichloropyridine-2-carboxylate (5.00 g, 24.3 mmol) in methanol (40 mL) was added sodium borohydride (1.80 g, 48.5 mmol), after which the reaction was warmed to room temperature and stirred at that temperature for two hours. The reaction mixture was then quenched by the addition of water (5 mL), concentrated to a residue, reconstituted in water (60 mL), extracted with ethyl acetate (2 x 60 mL), dried (magnesium sulfate), filtered and concentrated to afford (3,5-dichloropyridin-2-yl)methanol (2.90 g, 16.3 mmol, 67% yield ) as a viscous oil. This material was used in the subsequent step without any purification.[00378] To a 0 °C solution of (3,5-dichloropyridin-2-yl)methanol (2.90 g, 16.3 mmol) in dichloromethane (50 mL) was added thionyl chloride (2.31 g, 19.6 mmol) dropwise, after which the reaction mixture was allowed to warm up to room temperature and stirred at that temperature for two hours. The reaction mixture was washed by the addition of saturated sodium bicarbonate solution (1 x 40 mL) and the organic layer was separated, dried (sodium sulfate), filtered and concentrated to a residue. Purification was achieved by silica gel chromatography using 9% ethyl acetate in hexanes to afford 3,5-dichloro-2- (chloromethyl)pyridine (2.40 g, 12.2 mmol, 75% yield) as an off-white solid. NMR (300 MHz, CDC13) δ (ppm): 8.36 (s, 1H), 7.56 (s, 1H), 4.66 (s, 2H).
References:
WO2012/88469,2012,A1 Location in patent:Page/Page column 153-154
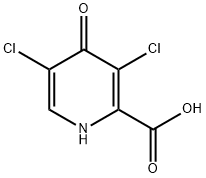
858443-51-3
0 suppliers
inquiry

70151-22-3
12 suppliers
$236.81/1gm:
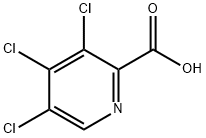
5439-04-3
41 suppliers
inquiry

70151-22-3
12 suppliers
$236.81/1gm:

200711-45-1
1 suppliers
inquiry

70151-22-3
12 suppliers
$236.81/1gm: