
3,5-difluorobenzophenone synthesis
- Product Name:3,5-difluorobenzophenone
- CAS Number:179113-89-4
- Molecular formula:C13H8F2O
- Molecular Weight:218.2
Yield: 75%
Reaction Conditions:
with copper(l) iodide;silver carbonate in N,N-dimethyl-formamide at 120; for 24 h;
Steps:
General procedure for the synthesis of Biaryl ketones from thiolester
General procedure: Thiolester (0.25 mmol), arylboronic acids (0.375 mmol), CuI (20 mol%, 10 mg), Ag2CO3 (5 mol%, 4 mg) and DMF (3 ml) were taken in a 25 ml round bottomed flask. The reaction mixture was heated at 120 C for 8-24 h. The reaction monitored using TLC. Upon completion, the reaction mixture was diluted with water and extracted with DCM (3x10 ml). The combined organic layer was dried over anhydrous sodium sulphate and concentrated under reduced pressure. The resulting ketone was purified was purified using silica gel column chromatography (EtOAc and petroleum ether 60-80 C).
References:
Ghosh, Prasanjit;Ganguly, Bhaskar;Das, Sajal;Perl, Eliyahu [Tetrahedron Letters,2017,vol. 58,# 28,p. 2751 - 2756]

108-86-1
496 suppliers
$10.00/5g
201230-82-2
1 suppliers
inquiry
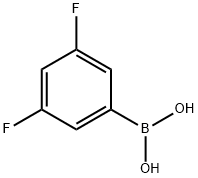
156545-07-2
401 suppliers
$5.00/1g

179113-89-4
69 suppliers
$14.00/1g
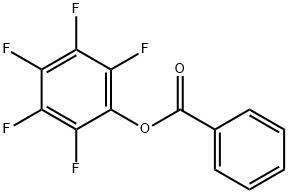
1548-84-1
37 suppliers
$24.00/1g

156545-07-2
401 suppliers
$5.00/1g

179113-89-4
69 suppliers
$14.00/1g
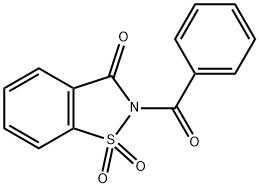
37952-93-5
0 suppliers
inquiry

156545-07-2
401 suppliers
$5.00/1g

179113-89-4
69 suppliers
$14.00/1g

