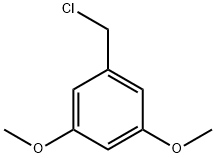
3,5-Dimethoxybenzyl chloride synthesis
- Product Name:3,5-Dimethoxybenzyl chloride
- CAS Number:6652-32-0
- Molecular formula:C9H11ClO2
- Molecular Weight:186.64
705-76-0
311 suppliers
$10.00/1g

6652-32-0
114 suppliers
$25.00/250mg
Yield: 97%
Reaction Conditions:
with pyridine;thionyl chloride in diethyl ether at 20;Cooling with ice;
Steps:
3,5-Dimethoxybenzyl chloride (5)
A round-bottomed flask (500 mL) equipped with magnetic stirring bar and addition funnel was charged with 4 (16.8 g, 100 mmol), pyridine (0.5 mL) and Et2O (250 mL). The mixture was cooled in an ice bath while the funnel was charged with SOCl2 (14.75 g, 1.25 equiv). The addition was made dropwise over 30 min. The ice bath was removed and the mixture was allowed to stir at rt for 12 h. The mixture was poured into H2O (500 mL) and extracted with Et2O (3 X 100 mL). The extracts were combined and washed H2O (2 X 200 mL) followed by brine (200 mL). After drying over anhydrous MgSO4 the solution was rotary evaporated leaving compound 5 an off-white solid that required no further purification (18.1 g, 97%). Mp 40-42 °C [lit.Adams 46 °C]. 1H NMR (CDCl3): δ 6.53 (d, J = 2.2 Hz, 2H), 6.40 (t, J = 2.2 Hz, 1H), 4.49 (s, 2H), 3.78 (s, 6H); 13C NMR (CDCl3): δ 161.14, 139.70, 106.65, 100.63, 55.53, 46.49.
References:
Davis, Matthew C.;Groshens, Thomas J. [Tetrahedron Letters,2012,vol. 53,# 27,p. 3521 - 3523] Location in patent:supporting information; experimental part

2150-37-0
293 suppliers
$6.00/5g

6652-32-0
114 suppliers
$25.00/250mg
7311-34-4
320 suppliers
$10.00/1g

6652-32-0
114 suppliers
$25.00/250mg

1132-21-4
354 suppliers
$10.00/5g

6652-32-0
114 suppliers
$25.00/250mg
99-10-5
516 suppliers
$5.00/5g

6652-32-0
114 suppliers
$25.00/250mg