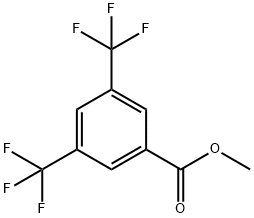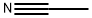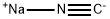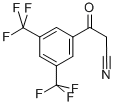
3,5-Trifluromethylbenzoylacetonitrile synthesis
- Product Name:3,5-Trifluromethylbenzoylacetonitrile
- CAS Number:267880-81-9
- Molecular formula:C11H5F6NO
- Molecular Weight:281.15
Yield:267880-81-9 95%
Reaction Conditions:
with sodium hydride in tetrahydrofuran at 20;Inert atmosphere;
Steps:
4.1.1. 3-(3,5-bis(trifluoromethyl)phenyl)-3-oxopropanenitrile (2)
To dried and nitrogen-replaced two-neck round bottom flaskwere added the actonitrile (9.0 g, 220.4 mmol) and sodium hydride(7.9 g, 330.9 mmol). The mixture was diluted with anhydrous THF(200 mL) and methyl 3, 5-bis(trifluoromethyl)benzoate (1.3 g,110.3 mmol) was added to the solution. The reaction was stirredovernight at rt. After TLC monitoring, the mixturewas concentratedin vacuo. The residue was diluted with water, acidified to pH of 1e2with 2 N hydrochloric acid and stirred in an ice bath for 1 h. Whitesolid was precipitated and separated by suction filtration. The filtercake was washed with a small amount of water and dried to affordthe product. The product was directly used for the next stepwithout purification. Yield: 95%; 1H NMR (500 MHz, DMSO-d6)d 8.50 (d, J 8.14 Hz, 2H), 8.45e8.22 (m, 1H), 4.92 (s, 1H).
References:
Qu, Bingxue;Xu, Yongjin;Lu, Yang;Zhuang, Weihao;Jin, Xinxin;Shi, Qiuqiu;Yan, Shike;Guo, Yu;Shen, Zheyuan;Che, Jinxin;Wu, Yize;Tong, Lexian;Dong, Xiaowu;Yang, Haiyan [European Journal of Medicinal Chemistry,2022,vol. 235,art. no. 114257]